Distinguishing Selection Bias and Confounding Bias in Comparative Effectiveness Research
- PMID: 24309675
- PMCID: PMC4043938
- DOI: 10.1097/MLR.0000000000000011
Distinguishing Selection Bias and Confounding Bias in Comparative Effectiveness Research
Abstract
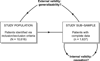
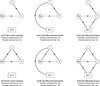
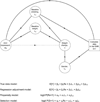
Comparative effectiveness research (CER) aims to provide patients and physicians with evidence-based guidance on treatment decisions. As researchers conduct CER they face myriad challenges. Although inadequate control of confounding is the most-often cited source of potential bias, selection bias that arises when patients are differentially excluded from analyses is a distinct phenomenon with distinct consequences: confounding bias compromises internal validity, whereas selection bias compromises external validity. Despite this distinction, however, the label "treatment-selection bias" is being used in the CER literature to denote the phenomenon of confounding bias. Motivated by an ongoing study of treatment choice for depression on weight change over time, this paper formally distinguishes selection and confounding bias in CER. By formally distinguishing selection and confounding bias, this paper clarifies important scientific, design, and analysis issues relevant to ensuring validity. First is that the 2 types of biases may arise simultaneously in any given study; even if confounding bias is completely controlled, a study may nevertheless suffer from selection bias so that the results are not generalizable to the patient population of interest. Second is that the statistical methods used to mitigate the 2 biases are themselves distinct; methods developed to control one type of bias should not be expected to address the other. Finally, the control of selection and confounding bias will often require distinct covariate information. Consequently, as researchers plan future studies of comparative effectiveness, care must be taken to ensure that all data elements relevant to both confounding and selection bias are collected.
Figures
Similar articles
-
Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report--Part II.Value Health. 2009 Nov-Dec;12(8):1053-61. doi: 10.1111/j.1524-4733.2009.00601.x. Epub 2009 Sep 10. Value Health. 2009. PMID: 19744292
-
Introduction.J Manag Care Pharm. 2011 Nov-Dec;17(9 Suppl A):S03-4. doi: 10.18553/jmcp.2011.17.s9-a.S03. J Manag Care Pharm. 2011. PMID: 22074667 Free PMC article.
-
Pre-study feasibility and identifying sensitivity analyses for protocol pre-specification in comparative effectiveness research.J Comp Eff Res. 2014 May;3(3):259-70. doi: 10.2217/cer.14.16. J Comp Eff Res. 2014. PMID: 24969153
-
A review of covariate selection for non-experimental comparative effectiveness research.Pharmacoepidemiol Drug Saf. 2013 Nov;22(11):1139-45. doi: 10.1002/pds.3506. Epub 2013 Sep 5. Pharmacoepidemiol Drug Saf. 2013. PMID: 24006330 Free PMC article. Review.
-
Core Concepts in Pharmacoepidemiology: Quantitative Bias Analysis.Pharmacoepidemiol Drug Saf. 2024 Oct;33(10):e70026. doi: 10.1002/pds.70026. Pharmacoepidemiol Drug Saf. 2024. PMID: 39375940 Review.
Cited by
-
Estimating individualized treatment rules for multicategory type 2 diabetes treatments using electronic health records.Stat Interface. 2023;16(4):505-515. doi: 10.4310/22-sii739. Epub 2023 Apr 14. Stat Interface. 2023. PMID: 38344146 Free PMC article.
-
Massage Compared with Massage Plus Acupuncture for Breast Cancer Patients Undergoing Reconstructive Surgery.J Altern Complement Med. 2020 Jul;26(7):602-609. doi: 10.1089/acm.2019.0479. J Altern Complement Med. 2020. PMID: 32673082 Free PMC article. Clinical Trial.
-
Toward a better understanding about real-world evidence.Eur J Hosp Pharm. 2022 Jan;29(1):8-11. doi: 10.1136/ejhpharm-2021-003081. Epub 2021 Dec 2. Eur J Hosp Pharm. 2022. PMID: 34857642 Free PMC article. Review.
-
Causal inference in studies of preterm babies: a simulation study.BJOG. 2018 May;125(6):686-692. doi: 10.1111/1471-0528.14942. Epub 2017 Oct 30. BJOG. 2018. PMID: 28941068 Free PMC article.
-
Directed acyclic graphs: a tool for causal studies in paediatrics.Pediatr Res. 2018 Oct;84(4):487-493. doi: 10.1038/s41390-018-0071-3. Epub 2018 Jun 4. Pediatr Res. 2018. PMID: 29967527 Free PMC article. Review.
References
-
- Steinbrook R. Health care and the American Recovery and Reinvestment Act. The New England Journal of Medicine. 2009;360(11):1057–1060. - PubMed
-
- Federal Coordinating Council for Comparative Effectiveness Research (U.S.), United States. President., United States. Congress., United States. Dept. of Health and Human Services. Report to the President and the Congress. Washington, DC: US Dept. of Health and Human Services; 2009.
-
- Patient-Centered Outcomes Research Institute. http://www.pcori.org/.
-
- Rothman K, Greenland S, Lash TL. Modern Epidemiology. N/A. 3 ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
-
- Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

