Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder
- PMID: 24309905
- PMCID: PMC3988542
- DOI: 10.1038/npp.2013.334
Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder
Abstract
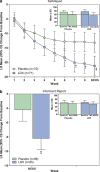
Evaluate lisdexamfetamine dimesylate (LDX) augmentation of antidepressant monotherapy for executive dysfunction in partially or fully remitted major depressive disorder (MDD). This randomized, placebo-controlled study (NCT00985725) enrolled 143 adults (18-55 years) with mild MDD (Montgomery-Åsberg Depression Rating Scale (MADRS) score ≤ 18) and executive dysfunction (Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) Self-Report Global Executive Composite (GEC) T score ≥ 60) on stable antidepressant monotherapy for ≥ 8 weeks. After 2 weeks of screening, participants were randomized to 9 weeks of double-blind LDX (20-70 mg/day) or placebo augmentation, followed by 2 weeks of single-blind placebo. The primary end point was change from baseline to week 9/end of study (EOS) in BRIEF-A Self-Report GEC T score; secondary assessments included the BRIEF-A Informant Report, MADRS, and treatment-emergent adverse events (TEAEs). Of 143 randomized participants, 119 completed double-blind treatment (placebo, n=59; LDX, n=60). Mean ± standard deviation (SD) BRIEF-A GEC T scores decreased from baseline (placebo, 74.2 ± 8.88; LDX, 76.8 ± 9.66) to week 9/EOS (placebo, 61.4 ± 14.61; LDX, 55.2 ± 16.15); the LS mean (95% CI) treatment difference significantly favored LDX (-8.0 (-12.7, -3.3); P=0.0009). The LS mean (95% CI) treatment difference for MADRS total score also significantly favored LDX (-1.9 (-3.7, 0.0); P=0.0465). TEAE rates were 73.6% with placebo and 78.9% with LDX; serious TEAE rates were 4.2 and 2.8%. In this trial, LDX augmentation significantly improved executive dysfunction and depressive symptoms in participants with mild MDD. The safety profile of LDX was consistent with prior studies in adults with attention-deficit/hyperactivity disorder.
Figures
Similar articles
-
Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: Results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies.J Affect Disord. 2016 Dec;206:151-160. doi: 10.1016/j.jad.2016.07.006. Epub 2016 Jul 5. J Affect Disord. 2016. PMID: 27474961 Clinical Trial.
-
Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design.Behav Brain Funct. 2010 Jun 24;6:34. doi: 10.1186/1744-9081-6-34. Behav Brain Funct. 2010. PMID: 20576091 Free PMC article. Clinical Trial.
-
Self-Reported quality of life in adults with attention-deficit/hyperactivity disorder and executive function impairment treated with lisdexamfetamine dimesylate: a randomized, double-blind, multicenter, placebo-controlled, parallel-group study.BMC Psychiatry. 2013 Oct 9;13:253. doi: 10.1186/1471-244X-13-253. BMC Psychiatry. 2013. PMID: 24106804 Free PMC article. Clinical Trial.
-
The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d-amphetamine, for the treatment of attention-deficit/hyperactivity disorder in children and adults.Clin Ther. 2009 Jan;31(1):142-76. doi: 10.1016/j.clinthera.2009.01.015. Clin Ther. 2009. PMID: 19243715 Review.
-
Dextromethorphan-Bupropion for the Treatment of Depression: A Systematic Review of Efficacy and Safety in Clinical Trials.CNS Drugs. 2023 Oct;37(10):867-881. doi: 10.1007/s40263-023-01032-5. Epub 2023 Oct 4. CNS Drugs. 2023. PMID: 37792265
Cited by
-
Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments.Can J Psychiatry. 2016 Sep;61(9):540-60. doi: 10.1177/0706743716659417. Epub 2016 Aug 2. Can J Psychiatry. 2016. PMID: 27486148 Free PMC article. Review.
-
Adult ADHD and comorbid disorders: clinical implications of a dimensional approach.BMC Psychiatry. 2017 Aug 22;17(1):302. doi: 10.1186/s12888-017-1463-3. BMC Psychiatry. 2017. PMID: 28830387 Free PMC article. Review.
-
Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment.Can J Psychiatry. 2014 Dec;59(12):649-54. doi: 10.1177/070674371405901206. Can J Psychiatry. 2014. PMID: 25702365 Free PMC article. Review. No abstract available.
-
Novel pharmacologic treatment in acute binge eating disorder - role of lisdexamfetamine.Neuropsychiatr Dis Treat. 2016 Apr 18;12:833-41. doi: 10.2147/NDT.S80881. eCollection 2016. Neuropsychiatr Dis Treat. 2016. PMID: 27143885 Free PMC article. Review.
-
Ketamine as an antidepressant: overview of its mechanisms of action and potential predictive biomarkers.Ther Adv Psychopharmacol. 2020 May 11;10:2045125320916657. doi: 10.1177/2045125320916657. eCollection 2020. Ther Adv Psychopharmacol. 2020. PMID: 32440333 Free PMC article.
References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. American Psychiatric Association: Washington, DC; 2000.
-
- Arnsten AF. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. - PubMed
-
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. - PubMed
-
- Brooks BL, Sherman EM. Computerized neuropsychological testing to rapidly evaluate cognition in pediatric patients with neurologic disorders. J Child Neurol. 2012;27:982–991. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

