Recognition of lipopolysaccharide pattern by TLR4 complexes
- PMID: 24310172
- PMCID: PMC3880462
- DOI: 10.1038/emm.2013.97
Recognition of lipopolysaccharide pattern by TLR4 complexes
Abstract
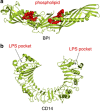
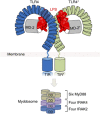
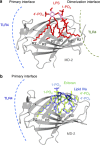
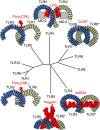
Lipopolysaccharide (LPS) is a major component of the outer membrane of Gram-negative bacteria. Minute amounts of LPS released from infecting pathogens can initiate potent innate immune responses that prime the immune system against further infection. However, when the LPS response is not properly controlled it can lead to fatal septic shock syndrome. The common structural pattern of LPS in diverse bacterial species is recognized by a cascade of LPS receptors and accessory proteins, LPS binding protein (LBP), CD14 and the Toll-like receptor4 (TLR4)-MD-2 complex. The structures of these proteins account for how our immune system differentiates LPS molecules from structurally similar host molecules. They also provide insights useful for discovery of anti-sepsis drugs. In this review, we summarize these structures and describe the structural basis of LPS recognition by LPS receptors and accessory proteins.
Figures

References
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
-
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. - PubMed
-
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
