Convergent diversity-oriented side-chain macrocyclization scan for unprotected polypeptides
- PMID: 24310320
- PMCID: PMC3935340
- DOI: 10.1039/c3ob42168f
Convergent diversity-oriented side-chain macrocyclization scan for unprotected polypeptides
Abstract
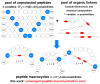
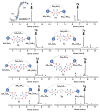
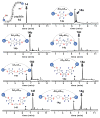
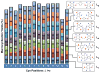
Here we describe a general synthetic platform for side-chain macrocyclization of an unprotected peptide library based on the SNAr reaction between cysteine thiolates and a new generation of highly reactive perfluoroaromatic small molecule linkers. This strategy enabled us to simultaneously "scan" two cysteine residues positioned from i, i + 1 to i, i + 14 sites in a polypeptide, producing 98 macrocyclic products from reactions of 14 peptides with 7 linkers. A complementary reverse strategy was developed; cysteine residues within the polypeptide were first modified with non-bridging perfluoroaryl moieties and then commercially available dithiol linkers were used for macrocyclization. The highly convergent, site-independent, and modular nature of these two strategies coupled with the unique chemoselectivity of a SNAr transformation allows for the rapid diversity-oriented synthesis of hybrid macrocyclic peptide libraries with varied chemical and structural complexities.
Figures

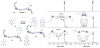
References
-
- Craik DJ. Science. 2006;311:1563–1564. - PubMed
-
-
Selected reviews: Lambert NJ, Mitchell JP, Roberts KD. J Chem Soc, Perkin Trans. 2001;1:471–484.Davies JS. J Pept Sci. 2003;9:471–501.White CJ, Yudin AK. Nature Chem. 2011;3:509–524.
-
-
-
Representative examples: Timmerman P, Beld J, Pujik WC, Meloen RH. Chem Bio Chem. 2005;6:821–824.Muppidi A, Doi K, Edwardraja S, Drake EJ, Gulick AM, Wang HG, Lin Q. J Am Chem Soc. 2012;134:14734–14737.Heinis C, Rutherford T, Freund S, Winter G. Nature Chem Bio. 2009;5:502–507.Smeenk LEJ, Dailly N, Hiemstra H, van Maarseveen JH, Timmerman P. Org Lett. 2012;14:1194–1197.Jo H, Meinhardt N, Wu Y, Kulkarni S, Hu X, Low KE, Davies PL, DeGrado WF, Greenbaum DC. J Am Chem Soc. 2012;134:17704–17713.Kawakami T, Ishizawa T, Fujino T, Reid PC, Suga H, Murakami H. ACS Chem Bio. 2013;8:1205–1214.
-
-
-
Representative examples, olefin metathesis: Miller SJ, Blackwell HE, Grubbs RH. J Am Chem Soc. 1996;118:9606–9614.Kim YW, Grossmann TN, Verdine GL. Nature Prot. 2011;6:761–771.Kim YW, Kutchukian PS, Verdine GL. Org Lett. 2010;12:3046–3049.Kim YW, Verdine GL. Bioorg & Med Chem Lett. 2009;19:2533–2536.Bautista AD, Appelbaum JS, Craig CJ, Michel J, Schepartz A. J Am Chem Soc. 2010;132:2904–2906.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Nature. 2009;462:182–188.Reichwein JF, Wels B, Kruijtzer JAW, Versluis C, Liskamp RMJ. Angew Chem, Int Ed. 1999;38:3684–3687.Yeo DJ, Warriner SL, Wilson AJ. Chem Commun. 2013;49:9131–9133.aryl cross-coupling: Dong H, Limberakis C, Price D, James K. Chem Commun. 2012;48:11644–11646.Meyer FM, Collins JC, Borin B, Bradow J, Liras S, Limberakis C, Mathiowetz AM, Philippe L, Price D, Song K, James K. J Org Chem. 2012;77:3099–3114.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

