The pharmacokinetics and pharmacodynamics of iron preparations
- PMID: 24310424
- PMCID: PMC3857035
- DOI: 10.3390/pharmaceutics3010012
The pharmacokinetics and pharmacodynamics of iron preparations
Abstract
Standard approaches are not appropriate when assessing pharmacokinetics of iron supplements due to the ubiquity of endogenous iron, its compartmentalized sites of action, and the complexity of the iron metabolism. The primary site of action of iron is the erythrocyte, and, in contrast to conventional drugs, no drug-receptor interaction takes place. Notably, the process of erythropoiesis, i.e., formation of new erythrocytes, takes 3-4 weeks. Accordingly, serum iron concentration and area under the curve (AUC) are clinically irrelevant for assessing iron utilization. Iron can be administered intravenously in the form of polynuclear iron(III)-hydroxide complexes with carbohydrate ligands or orally as iron(II) (ferrous) salts or iron(III) (ferric) complexes. Several approaches have been employed to study the pharmacodynamics of iron after oral administration. Quantification of iron uptake from radiolabeled preparations by the whole body or the erythrocytes is optimal, but alternatively total iron transfer can be calculated based on known elimination rates and the intrinsic reactivity of individual preparations. Degradation kinetics, and thus the safety, of parenteral iron preparations are directly related to the molecular weight and the stability of the complex. High oral iron doses or rapid release of iron from intravenous iron preparations can saturate the iron transport system, resulting in oxidative stress with adverse clinical and subclinical consequences. Appropriate pharmacokinetics and pharmacodynamics analyses will greatly assist our understanding of the likely contribution of novel preparations to the management of anemia.
Figures



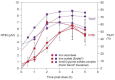
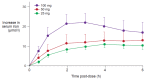
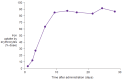
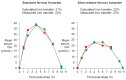
References
-
- Gotloib L., Silverberg D., Fudin R., Shostak A. Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J. Nephrol. 2006;19:161–167. - PubMed
-
- Nanas P.N., Matsouka C., Karageorgopoulos D., Leonti A., Tsolakis E., Drakos S.G., Tsagalou E.P., Maroulidis G.D., Alexopoulos G.P., Kanakakis J.E., et al. Etiology of anemia in patients with advanced heart failure. J. Amer. Coll. Cardiol. 2006;48:2485–2489. - PubMed
-
- Kalantar-Zadeh K., Streja E., Miller J.E., Nissenson A.R. Intravenous iron versus erythropoiesis-stimulating agents: friends or foes in treating chronic kidney disease anemia? Adv. Chronic Kidney Dis. 2009;16:143–151. - PubMed
-
- Schümann K., Classen H.G., Hages M., Prinz-Langenohl R., Pietrzik K., Biesalski H.K. Bioavailability of oral vitamins, minerals, and trace elements in perspective. Drug Res. 1997;47:369–380. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

