Structure of human apurinic/apyrimidinic endonuclease 1 with the essential Mg2+ cofactor
- PMID: 24311596
- PMCID: PMC3852660
- DOI: 10.1107/S0907444913027042
Structure of human apurinic/apyrimidinic endonuclease 1 with the essential Mg2+ cofactor
Abstract
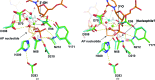
Apurinic/apyrimidinic endonuclease 1 (APE1) mediates the repair of abasic sites and other DNA lesions and is essential for base-excision repair and strand-break repair pathways. APE1 hydrolyzes the phosphodiester bond at abasic sites, producing 5'-deoxyribose phosphate and the 3'-OH primer needed for repair synthesis. It also has additional repair activities, including the removal of 3'-blocking groups. APE1 is a powerful enzyme that absolutely requires Mg2+, but the stoichiometry and catalytic function of the divalent cation remain unresolved for APE1 and for other enzymes in the DNase I superfamily. Previously reported structures of DNA-free APE1 contained either Sm3+ or Pb2+ in the active site. However, these are poor surrogates for Mg2+ because Sm3+ is not a cofactor and Pb2+ inhibits APE1, and their coordination geometry is expected to differ from that of Mg2+. A crystal structure of human APE1 was solved at 1.92 Å resolution with a single Mg2+ ion in the active site. The structure reveals ideal octahedral coordination of Mg2+ via two carboxylate groups and four water molecules. One residue that coordinates Mg2+ directly and two that bind inner-sphere water molecules are strictly conserved in the DNase I superfamily. This structure, together with a recent structure of the enzyme-product complex, inform on the stoichiometry and the role of Mg2+ in APE1-catalyzed reactions.
Keywords: apurinic/apyrimidinic DNA; base-excision repair; nucleases; phosphoryl transfer.
Figures

References
-
- Abbotts, R. & Madhusudan, S. (2010). Cancer Treat. Rev. 36, 425–435. - PubMed
-
- Adhikari, S., Choudhury, S., Mitra, P. S., Dubash, J. J., Sajankila, S. P. & Roy, R. (2008). Anticancer Agents Med. Chem. 8, 351–357. - PubMed
-
- Barzilay, G., Mol, C. D., Robson, C. N., Walker, L. J., Cunningham, R. P., Tainer, J. A. & Hickson, I. D. (1995). Nature Struct. Biol. 2, 561–568. - PubMed
-
- Beernink, P. T., Segelke, B. W., Hadi, M. Z., Erzberger, J. P., Wilson, D. M. III & Rupp, B. (2001). J. Mol. Biol. 307, 1023–1034. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

