Clinical and molecular genetics of the phosphodiesterases (PDEs)
- PMID: 24311737
- PMCID: PMC3963262
- DOI: 10.1210/er.2013-1053
Clinical and molecular genetics of the phosphodiesterases (PDEs)
Abstract
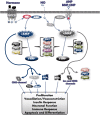
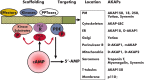
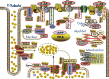
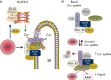
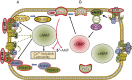
Cyclic nucleotide phosphodiesterases (PDEs) are enzymes that have the unique function of terminating cyclic nucleotide signaling by catalyzing the hydrolysis of cAMP and GMP. They are critical regulators of the intracellular concentrations of cAMP and cGMP as well as of their signaling pathways and downstream biological effects. PDEs have been exploited pharmacologically for more than half a century, and some of the most successful drugs worldwide today affect PDE function. Recently, mutations in PDE genes have been identified as causative of certain human genetic diseases; even more recently, functional variants of PDE genes have been suggested to play a potential role in predisposition to tumors and/or cancer, especially in cAMP-sensitive tissues. Mouse models have been developed that point to wide developmental effects of PDEs from heart function to reproduction, to tumors, and beyond. This review brings together knowledge from a variety of disciplines (biochemistry and pharmacology, oncology, endocrinology, and reproductive sciences) with emphasis on recent research on PDEs, how PDEs affect cAMP and cGMP signaling in health and disease, and what pharmacological exploitations of PDEs may be useful in modulating cyclic nucleotide signaling in a way that prevents or treats certain human diseases.
Figures




References
-
- Ashman DF, Lipton R, Melicow MM, Price TD. Isolation of adenosine 3′, 5′-monophosphate and guanosine 3′, 5′-monophosphate from rat urine. Biochem Biophys Res Commun. 1963;11:330–334 - PubMed
-
- Butcher RW, Sutherland EW. Adenosine 3′,5′-phosphate in biological materials. I. Purification and properties of cyclic 3′,5′-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3′,5′-phosphate in human urine. J Biol Chem. 1962;237:1244–1250 - PubMed
-
- Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem. 1958;232:1077–1091 - PubMed
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520 - PubMed
-
- Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev. 2011;91:651–690 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

