Upregulation of zinc transporter 2 in the blood-CSF barrier following lead exposure
- PMID: 24311739
- PMCID: PMC3928640
- DOI: 10.1177/1535370213509213
Upregulation of zinc transporter 2 in the blood-CSF barrier following lead exposure
Abstract
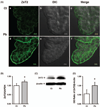
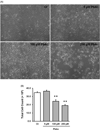
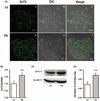
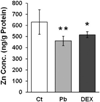
Zinc (Zn) is an essential element for normal brain function; an abnormal Zn homeostasis in brain and the cerebrospinal fluid (CSF) has been implied in the etiology of Alzheimer's disease (AD). However, the mechanisms that regulate Zn transport in the blood-brain interface remain unknown. This study was designed to investigate Zn transport by the blood-CSF barrier (BCB) in the choroid plexus, with a particular focus on Zn transporter-2 (ZnT2), and to understand if lead (Pb) accumulation in the choroid plexus disturbed the Zn regulatory function in the BCB. Confocal microscopy, quantitative PCR and western blot demonstrated the presence of ZnT2 in the choroidal epithelia; ZnT2 was primarily in cytosol in freshly isolated plexus tissues but more toward the peripheral membrane in established choroidal Z310 cells. Exposure of rats to Pb (single ip injection of 50 mg Pb acetate/kg) for 24 h increased ZnT2 fluorescent signals in plexus tissues by confocal imaging and protein expression by western blot. Similar results were obtained by in vitro experiments using Z310 cells. Further studies using cultured cells and a two-chamber Transwell device showed that Pb treatment significantly reduced the cellular Zn concentration and led to an increased transport of Zn across the BCB, the effect that may be due to the increased ZnT2 by Pb exposure. Taken together, these results indicate that ZnT2 is present in the BCB; Pb exposure increases the ZnT2 expression in choroidal epithelial cells by a yet unknown mechanism and as a result, more Zn ions may be deposited into the intracellular Zn pool, leading to a relative Zn deficiency state in the cytoplasm at the BCB.
Keywords: Z310 cells; Zinc (Zn); blood–CSF barrier; lead (Pb); zinc transporter 2 (ZnT2).
Figures


References
-
- Colvin RA, Davis N, Nipper RW, Carter PA. Zinc transport in the brain: routes of zinc influx and efflux in neurons. J Nutr. 2000;130:1484S–1487S. - PubMed
-
- Prasad AS. The role of zinc in brain and nerve functions. In: Connor, editor. Metals and oxidative damage in neurological disorders. New York, NY: Plenum Press; 1997. pp. 95–111.
-
- Takeda A. Zinc homeostasis and functions of zinc in the brain. BioMetals. 2001;14:343–351. - PubMed
-
- Kim YH, Park JH, Hong SH, Koh JY. Nonproteolytic neuroprotection by human recombinant tissue plasminogen activator. Science. 1999;284:647–650. - PubMed
-
- Kim EY, Koh JY, Kim YH, Sohn S, Joe E, Gwag BJ. Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. Eur J Neurosci. 1999;11:327–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

