Development of PLGA-based itraconazole injectable nanospheres for sustained release
- PMID: 24311942
- PMCID: PMC3839800
- DOI: 10.2147/IJN.S54040
Development of PLGA-based itraconazole injectable nanospheres for sustained release
Abstract
Purpose: Itraconazole (ITZ) is a synthetic triazole antifungal agent, which is widely used for treatment and prevention of fungal infections. The purpose of this study is to develop ITZ-loaded poly(lactic-co-glycolic acid) (PLGA) nanospheres (PLGA-ITZ-NS) as a new sustained-release formulation for intravenous ITZ administration.
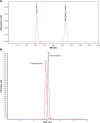
Materials and methods: PLGA-ITZ-NS were prepared by a nanoprecipitation method and optimized by modifying the surfactant poloxamer 188 concentration and PLGA:ITZ ratio. Their physicochemical properties, including size, zeta potential, external morphology and encapsulation efficiency, were characterized by dynamic light scattering (DLS), scanning electron microscopy (SEM) and high performance liquid chromatography (HPLC). The effect of the different selected lyoprotectants with various concentrations on NS particles size and surface charge were also assessed. Rapid and sensitive HPLC and liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods were developed to determine ITZ concentrations in formulation and in rat plasma, respectively. Pharmacokinetics of the optimum PLGA-ITZ-NS formulation was compared with the former commercial Sporanox® injection formulation using rats as the animal model. Noncompartmental pharmacokinetic parameters were obtained by WinNonlin® software.
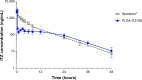
Results: Optimal PLGA-ITZ-NS had a mean particle size of about 200 nm with a high homogeneity (polydispersity index ≈0.2), favorable zeta potential (approximately -20 to -30 mV) and encapsulation efficiency (72%). In addition, 2% w/v sucrose was selected as a lyoprotectant for NS freeze-drying. The newly developed LC-MS/MS assay was validated and found to be accurate and precise. The in vivo study showed that the NS formulation has a similar systemic bioavailability to Sporanox® while providing a sustained plasma level (> 100 ng/mL) for up to 24 hours after intravenous administration.
Conclusion: Our newly developed PLGA-ITZ-NS has shown great sustained release and comparable bioavailability with Sporanox®, therefore having the potential to be an alternative injectable formulation of ITZ.
Keywords: PLGA; intravenous injection; itraconazole; nanoparticle; pharmacokinetics; poly(lactic-co-glycolic acid); sustained release.
Figures

Similar articles
-
Itraconazole-loaded poly(lactic-co-glycolic) acid nanoparticles for improved antifungal activity.Nanomedicine (Lond). 2010 Sep;5(7):1037-50. doi: 10.2217/nnm.10.68. Nanomedicine (Lond). 2010. PMID: 20874019
-
Antifungal efficacy of Itraconazole loaded PLGA-nanoparticles stabilized by vitamin-E TPGS: In vitro and ex vivo studies.J Microbiol Methods. 2019 Jun;161:87-95. doi: 10.1016/j.mimet.2019.01.020. Epub 2019 Feb 7. J Microbiol Methods. 2019. PMID: 30738109
-
Itraconazole Loaded Micelle Based on Methoxy Poly(Ethylene Glycol)-Poly(D, L-Lactic Acid) for Ocular Drug Delivery: In vitro and in vivo Evaluation.Int J Nanomedicine. 2025 May 22;20:6447-6462. doi: 10.2147/IJN.S521127. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40420914 Free PMC article.
-
Formulation optimization of etoposide loaded PLGA nanoparticles by double factorial design and their evaluation.Curr Drug Deliv. 2010 Jan;7(1):51-64. doi: 10.2174/156720110790396517. Curr Drug Deliv. 2010. PMID: 20044908 Review.
-
Injectable controlled release depots for large molecules.J Control Release. 2014 Sep 28;190:240-53. doi: 10.1016/j.jconrel.2014.05.057. Epub 2014 Jun 12. J Control Release. 2014. PMID: 24929039 Free PMC article. Review.
Cited by
-
Review on Nanomaterials and Nano-Scaled Systems for Topical and Systemic Delivery of Antifungal Drugs.J Multidiscip Healthc. 2022 Aug 27;15:1819-1840. doi: 10.2147/JMDH.S359282. eCollection 2022. J Multidiscip Healthc. 2022. PMID: 36060421 Free PMC article. Review.
-
Synthesis, characterization, and in vitro activity against Candida spp. of fluconazole encapsulated on cationic and conventional nanoparticles of poly(lactic-co-glycolic acid).Nanotechnol Sci Appl. 2017 May 16;10:95-104. doi: 10.2147/NSA.S96018. eCollection 2017. Nanotechnol Sci Appl. 2017. PMID: 28572725 Free PMC article.
-
Functional Nanocarriers for Delivering Itraconazole Against Fungal Intracellular Infections.Front Pharmacol. 2021 Jun 28;12:685391. doi: 10.3389/fphar.2021.685391. eCollection 2021. Front Pharmacol. 2021. PMID: 34262456 Free PMC article.
-
The evolution of antifungal therapy: Traditional agents, current challenges and future perspectives.Curr Res Microb Sci. 2025 Jan 11;8:100341. doi: 10.1016/j.crmicr.2025.100341. eCollection 2025. Curr Res Microb Sci. 2025. PMID: 39897698 Free PMC article. Review.
-
Investigating the Impact of Optimized Trans-Cinnamic Acid-Loaded PLGA Nanoparticles on Epithelial to Mesenchymal Transition in Breast Cancer.Int J Nanomedicine. 2022 Feb 18;17:733-750. doi: 10.2147/IJN.S345870. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35210772 Free PMC article.
References
-
- Willems L, van der Geest R, de Beule K. Itraconazole oral solution and intravenous formulations: a review of pharmacokinetics and pharmacodynamics. J Clin Pharm Ther. 2001;26(3):159–169. - PubMed
-
- ClinicalTrials.gov [webpage on the Internet] Itraconazole clinical trials Available from: http://clinicaltrials.gov/ct2/results?term=itraconazoleAccessed October 23, 2013
-
- Peeters J, Neeskens P, Tollenaere JP, Van Remoortere P, Brewster ME. Characterization of the interaction of 2-hydroxypropyl-beta-cyclodextrin with itraconazole at pH 2, 4, and 7. J Pharm Sci. 2002;91(6):1414–1422. - PubMed
-
- Kim JK, Park JS, Kim CK. Development of a binary lipid nanoparticles formulation of itraconazole for parenteral administration and controlled release. Int J Pharm. 2010;383(1–2):209–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

