Publication patterns of cancer cost-effectiveness studies presented at major conferences
- PMID: 24311947
- PMCID: PMC3851343
- DOI: 10.3747/co.20.1438
Publication patterns of cancer cost-effectiveness studies presented at major conferences
Abstract
Objective: To be useful to policymakers and stakeholders, cost-effectiveness analyses (ceas) should be published in a timely manner and without bias. The aims of the present study were to examine the time between conference abstract presentation and subsequent publication, to determine the factors associated with time to publication, to evaluate potential publication bias, and to examine discrepancies in the results between abstract and publication.
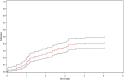
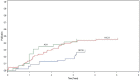
Methods: Abstracts of ceas presented at the annual meetings of the American Society of Clinical Oncology (asco), the American Society of Hematology (ash), and the International Society for Pharmacoeconomics and Outcomes Research (ispor) between 1997 and 2007 were reviewed. Time-to-event analysis was performed to assess the timeliness of publication and to examine factors associated with time to publication. Summary statistics were used to assess discrepancies in incremental cost-effectiveness ratios (icers) between abstract and publication.
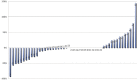
Results: Of 164 abstracts identified, 65 (39.6%) were subsequently published. The 1-, 2-, 3-, and 5-year publication rates were 12.8%, 25%, 34.2%, and 40.5% respectively. Abstracts were more likely to be published if presented at asco than at ispor (hazard ratio: 1.94; p = 0.038). There was no direct evidence of publication bias for abstracts with favourable icers. Comparing icers between abstracts and publications, the mean absolute difference was 23.8%; 50% of studies had a change in icer exceeding 10%.
Conclusions: Publication rates for ceas were low, and publication was not timely with respect to informing the decision-making process for funding. Abstract results often differed from publication results and cannot reliably be used in the decision-making process for funding.
Keywords: Cost-effectiveness analyses; abstracts; publication bias; time to publication.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

