Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells
- PMID: 24311969
- PMCID: PMC3845966
- DOI: 10.1088/1468-6996/14/2/025003
Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells
Abstract
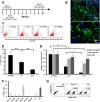
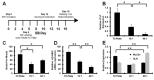
While adult heart muscle is the least regenerative of tissues, embryonic cardiomyocytes are proliferative, with embryonic stem (ES) cells providing an endless reservoir. In addition to secreted factors and cell-cell interactions, the extracellular microenvironment has been shown to play an important role in stem cell lineage specification, and understanding how scaffold elasticity influences cardiac differentiation is crucial to cardiac tissue engineering. Though previous studies have analyzed the role of the matrix elasticity on the function of differentiated cardiomyocytes, whether it affects the induction of cardiomyocytes from pluripotent stem cells is poorly understood. Here, we examined the role of matrix rigidity on the cardiac differentiation using mouse and human ES cells. Culture on polydimethylsiloxane (PDMS) substrates of varied monomer-to-crosslinker ratios revealed that rigid extracellular matrices promote a higher yield of de novo cardiomyocytes from undifferentiated ES cells. Using an genetically modified ES system that allows us to purify differentiated cardiomyocytes by drug selection, we demonstrate that rigid environments induce higher cardiac troponin T expression, beating rate of foci, and expression ratio of adult α- to fetal β- myosin heavy chain in a purified cardiac population. M-mode and mechanical interferometry image analyses demonstrate that these ES-derived cardiomyocytes display functional maturity and synchronization of beating when co-cultured with neonatal cardiomyocytes harvested from a developing embryo. Together, these data identify matrix stiffness as an independent factor that instructs not only the maturation of the already differentiated cardiomyocytes but also the induction and proliferation of cardiomyocytes from undifferentiated progenitors. Manipulation of the stiffness will help direct the production of functional cardiomyocytes en masse from stem cells for regenerative medicine purposes.
Keywords: Cardiac Differentiation; Drug-selected cardiomyocyte; Matrix elasticity; Mechanical interferometry; Pluripotent Embryonic Stem Cell; Synchronization.
Figures


Similar articles
-
Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages.Acta Biomater. 2018 Jan;65:44-52. doi: 10.1016/j.actbio.2017.10.032. Epub 2017 Oct 21. Acta Biomater. 2018. PMID: 29066419
-
Generation of functional murine cardiac myocytes from induced pluripotent stem cells.Circulation. 2008 Jul 29;118(5):507-17. doi: 10.1161/CIRCULATIONAHA.108.778795. Epub 2008 Jul 14. Circulation. 2008. PMID: 18625890
-
Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating.Curr Biol. 2013 Dec 2;23(23):2434-9. doi: 10.1016/j.cub.2013.10.057. Epub 2013 Nov 21. Curr Biol. 2013. PMID: 24268417 Free PMC article.
-
Cardiac specific differentiation of mouse embryonic stem cells.Cardiovasc Res. 2003 May 1;58(2):278-91. doi: 10.1016/s0008-6363(03)00248-7. Cardiovasc Res. 2003. PMID: 12757863 Review.
-
Embryonic stem cell transplantation: promise and progress in the treatment of heart disease.BioDrugs. 2008;22(6):361-74. doi: 10.2165/0063030-200822060-00003. BioDrugs. 2008. PMID: 18998754 Review.
Cited by
-
Acute frataxin knockdown in induced pluripotent stem cell-derived cardiomyocytes activates a type I interferon response.Dis Model Mech. 2023 May 1;16(5):dmm049497. doi: 10.1242/dmm.049497. Epub 2022 Oct 26. Dis Model Mech. 2023. PMID: 36107856 Free PMC article.
-
Use of bio-mimetic three-dimensional technology in therapeutics for heart disease.Bioengineered. 2014 May-Jun;5(3):193-7. doi: 10.4161/bioe.27751. Epub 2014 Jan 14. Bioengineered. 2014. PMID: 24637710 Free PMC article.
-
The Use of Pluripotent Stem Cell-Derived Organoids to Study Extracellular Matrix Development during Neural Degeneration.Cells. 2019 Mar 14;8(3):242. doi: 10.3390/cells8030242. Cells. 2019. PMID: 30875781 Free PMC article. Review.
-
Two dimensional electrophysiological characterization of human pluripotent stem cell-derived cardiomyocyte system.Sci Rep. 2017 Mar 7;7:43210. doi: 10.1038/srep43210. Sci Rep. 2017. PMID: 28266620 Free PMC article.
-
Unchain My Heart: Integrins at the Basis of iPSC Cardiomyocyte Differentiation.Stem Cells Int. 2019 Feb 13;2019:8203950. doi: 10.1155/2019/8203950. eCollection 2019. Stem Cells Int. 2019. PMID: 30906328 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
