NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field
- PMID: 24311985
- PMCID: PMC3849182
- DOI: 10.1371/journal.pbio.1001725
NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field
Abstract
The heart and head muscles share common developmental origins and genetic underpinnings in vertebrates, including humans. Parts of the heart and cranio-facial musculature derive from common mesodermal progenitors that express NKX2-5, ISL1, and TBX1. This ontogenetic kinship is dramatically reflected in the DiGeorge/Cardio-Velo-Facial syndrome (DGS/CVFS), where mutations of TBX1 cause malformations in the pharyngeal apparatus and cardiac outflow tract. Cardiac progenitors of the first heart field (FHF) do not require TBX1 and segregate precociously from common progenitors of the second heart field (SHF) and pharyngeal muscles. However, the cellular and molecular mechanisms that govern heart versus pharyngeal muscle specification within this lineage remain elusive. Here, we harness the simplicity of the ascidian larva to show that, following asymmetric cell division of common progenitors, NK4/NKX2-5 promotes GATAa/GATA4/5/6 expression and cardiac specification in the second heart precursors by antagonizing Tbx1/10-mediated inhibition of GATAa and activation of Collier/Olf/EBF (COE), the determinant of atrial siphon muscle (ASM) specification. Our results uncover essential regulatory connections between the conserved cardio-pharyngeal factor Tbx1/10 and muscle determinant COE, as well as a mutual antagonism between NK4 and Tbx1/10 activities upstream of GATAa and COE. The latter cross-antagonism underlies a fundamental heart versus pharyngeal muscle fate choice that occurs in a conserved lineage of cardio-pharyngeal progenitors. We propose that this basic ontogenetic motif underlies cardiac and pharyngeal muscle development and evolution in chordates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

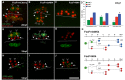
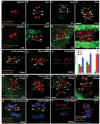
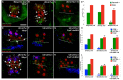
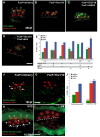

Similar articles
-
Collier/OLF/EBF-dependent transcriptional dynamics control pharyngeal muscle specification from primed cardiopharyngeal progenitors.Dev Cell. 2014 May 12;29(3):263-76. doi: 10.1016/j.devcel.2014.04.001. Epub 2014 May 1. Dev Cell. 2014. PMID: 24794633 Free PMC article.
-
Early chordate origins of the vertebrate second heart field.Science. 2010 Jul 30;329(5991):565-8. doi: 10.1126/science.1190181. Science. 2010. PMID: 20671188 Free PMC article.
-
Tbx1 affects asymmetric cardiac morphogenesis by regulating Pitx2 in the secondary heart field.Development. 2006 Apr;133(8):1565-73. doi: 10.1242/dev.02309. Development. 2006. PMID: 16556915
-
Regulation and evolution of cardiopharyngeal cell identity and behavior: insights from simple chordates.Curr Opin Genet Dev. 2015 Jun;32:119-28. doi: 10.1016/j.gde.2015.02.008. Epub 2015 Mar 25. Curr Opin Genet Dev. 2015. PMID: 25819888 Free PMC article. Review.
-
Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis.Cardiovasc Res. 2011 Jul 15;91(2):196-202. doi: 10.1093/cvr/cvr116. Epub 2011 Apr 15. Cardiovasc Res. 2011. PMID: 21498416 Free PMC article. Review.
Cited by
-
Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium.Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1446-51. doi: 10.1073/pnas.1424538112. Epub 2015 Jan 20. Proc Natl Acad Sci U S A. 2015. PMID: 25605943 Free PMC article.
-
Insulin-like genes in ascidians: findings in Ciona and hypotheses on the evolutionary origins of the pancreas.Genesis. 2015 Jan;53(1):82-104. doi: 10.1002/dvg.22832. Epub 2014 Nov 12. Genesis. 2015. PMID: 25378051 Free PMC article. Review.
-
Evaluation and rational design of guide RNAs for efficient CRISPR/Cas9-mediated mutagenesis in Ciona.Dev Biol. 2017 May 1;425(1):8-20. doi: 10.1016/j.ydbio.2017.03.003. Epub 2017 Mar 22. Dev Biol. 2017. PMID: 28341547 Free PMC article.
-
Heart fields and cardiac morphogenesis.Cold Spring Harb Perspect Med. 2014 Oct 1;4(10):a015750. doi: 10.1101/cshperspect.a015750. Cold Spring Harb Perspect Med. 2014. PMID: 25274757 Free PMC article. Review.
-
Ring Finger 149-Related Is an FGF/MAPK-Independent Regulator of Pharyngeal Muscle Fate Specification.Int J Mol Sci. 2023 May 16;24(10):8865. doi: 10.3390/ijms24108865. Int J Mol Sci. 2023. PMID: 37240211 Free PMC article.
References
-
- Jerome LA, Papaioannou VE (2001) DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet 27: 286–291. - PubMed
-
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, et al. (2001) TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 104: 619–629. - PubMed
-
- Kelly RG, Jerome-Majewska LA, Papaioannou VE (2004) The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet 13: 2829–2840. - PubMed
-
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, et al. (2001) Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410: 97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

