Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals
- PMID: 24311990
- PMCID: PMC3849189
- DOI: 10.1371/journal.pmed.1001566
Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals
Abstract
Background: The US Food and Drug Administration Amendments Act requires results from clinical trials of Food and Drug Administration-approved drugs to be posted at ClinicalTrials.gov within 1 y after trial completion. We compared the timing and completeness of results of drug trials posted at ClinicalTrials.gov and published in journals.
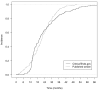
Methods and findings: We searched ClinicalTrials.gov on March 27, 2012, for randomized controlled trials of drugs with posted results. For a random sample of these trials, we searched PubMed for corresponding publications. Data were extracted independently from ClinicalTrials.gov and from the published articles for trials with results both posted and published. We assessed the time to first public posting or publishing of results and compared the completeness of results posted at ClinicalTrials.gov versus published in journal articles. Completeness was defined as the reporting of all key elements, according to three experts, for the flow of participants, efficacy results, adverse events, and serious adverse events (e.g., for adverse events, reporting of the number of adverse events per arm, without restriction to statistically significant differences between arms for all randomized patients or for those who received at least one treatment dose). From the 600 trials with results posted at ClinicalTrials.gov, we randomly sampled 50% (n = 297) had no corresponding published article. For trials with both posted and published results (n = 202), the median time between primary completion date and first results publicly posted was 19 mo (first quartile = 14, third quartile = 30 mo), and the median time between primary completion date and journal publication was 21 mo (first quartile = 14, third quartile = 28 mo). Reporting was significantly more complete at ClinicalTrials.gov than in the published article for the flow of participants (64% versus 48% of trials, p<0.001), efficacy results (79% versus 69%, p = 0.02), adverse events (73% versus 45%, p<0.001), and serious adverse events (99% versus 63%, p<0.001). The main study limitation was that we considered only the publication describing the results for the primary outcomes.
Conclusions: Our results highlight the need to search ClinicalTrials.gov for both unpublished and published trials. Trial results, especially serious adverse events, are more completely reported at ClinicalTrials.gov than in the published article.
Conflict of interest statement
IB is a member of the Editorial Board of
Figures
Similar articles
-
Comparison of serious adverse events posted at ClinicalTrials.gov and published in corresponding journal articles.BMC Med. 2015 Aug 14;13:189. doi: 10.1186/s12916-015-0430-4. BMC Med. 2015. PMID: 26269118 Free PMC article.
-
Public availability of results of observational studies evaluating an intervention registered at ClinicalTrials.gov.BMC Med. 2016 Jan 28;14:7. doi: 10.1186/s12916-016-0551-4. BMC Med. 2016. PMID: 26819213 Free PMC article.
-
Reporting of statistically significant results at ClinicalTrials.gov for completed superiority randomized controlled trials.BMC Med. 2016 Nov 30;14(1):192. doi: 10.1186/s12916-016-0740-1. BMC Med. 2016. PMID: 27899150 Free PMC article.
-
Comparison of reporting phase I trial results in ClinicalTrials.gov and matched publications.Invest New Drugs. 2017 Dec;35(6):827-833. doi: 10.1007/s10637-017-0510-8. Epub 2017 Sep 14. Invest New Drugs. 2017. PMID: 28905282 Review.
-
Non-publication of large randomized clinical trials: cross sectional analysis.BMJ. 2013 Oct 29;347:f6104. doi: 10.1136/bmj.f6104. BMJ. 2013. PMID: 24169943 Free PMC article. Review.
Cited by
-
Availability of results of clinical trials registered on EU Clinical Trials Register: cross sectional audit study.BMJ Med. 2024 Jan 12;3(1):e000738. doi: 10.1136/bmjmed-2023-000738. eCollection 2024. BMJ Med. 2024. PMID: 38274035 Free PMC article.
-
Opportunities for selective reporting of harms in randomized clinical trials: Selection criteria for non-systematic adverse events.Trials. 2019 Sep 5;20(1):553. doi: 10.1186/s13063-019-3581-3. Trials. 2019. PMID: 31488200 Free PMC article.
-
Availability of Results of Trials Studying Pancreatic Adenocarcinoma over the Past 10 Years.Oncologist. 2022 Nov 3;27(11):e849-e855. doi: 10.1093/oncolo/oyac156. Oncologist. 2022. PMID: 35983949 Free PMC article.
-
Quantifying Sex Bias in Clinical Studies at Scale With Automated Data Extraction.JAMA Netw Open. 2019 Jul 3;2(7):e196700. doi: 10.1001/jamanetworkopen.2019.6700. JAMA Netw Open. 2019. PMID: 31268541 Free PMC article.
-
Chinese Clinical Trial Registry 13-year data collection and analysis: geographic distribution, financial support, research phase, duration, and disease categories.Front Med (Lausanne). 2023 Oct 12;10:1203346. doi: 10.3389/fmed.2023.1203346. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37901406 Free PMC article.
References
-
- Chalmers I, Glasziou P (2009) Avoidable waste in the production and reporting of research evidence. Lancet 374: 86–89. - PubMed
-
- Antes G, Chalmers I (2003) Under-reporting of clinical trials is unethical. Lancet 361: 978–979. - PubMed
-
- Chalmers I (1990) Underreporting research is scientific misconduct. JAMA 263: 1405–1408. - PubMed
-
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR (1991) Publication bias in clinical research. Lancet 337: 867–872. - PubMed
-
- Liberati A (2004) An unfinished trip through uncertainties. BMJ 328: 531.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical