Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition
- PMID: 24312095
- PMCID: PMC3834540
- DOI: 10.3389/fimmu.2013.00385
Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition
Abstract
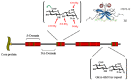
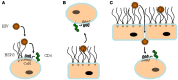
By targeting cells that provide protection against infection, HIV-1 causes acquired immunodeficiency syndrome. Infection starts when gp120, the viral envelope glycoprotein, binds to CD4 and to a chemokine receptor usually CCR5 or CXCR4. As many microorganisms, HIV-1 also interacts with heparan sulfate (HS), a complex group of cell surface associated anionic polysaccharides. It has been thought that this binding, occurring at a step prior to CD4 recognition, increases infectivity by pre-concentrating the virion particles at the cell surface. Early work, dating from before the identification of CCR5 and CXCR4, showed that a variety of HS mimetics bind to the gp120 V3 loop through electrostatic interactions, compete with cell surface associated HS to bind the virus and consequently, neutralize the infectivity of a number of T-cell line-adapted HIV-1 strains. However, progress made to better understand HIV-1 attachment and entry, coupled with the recent identification of additional gp120 regions mediating HS recognition, have considerably modified this view. Firstly, the V3 loop from CXCR4-using viruses is much more positively charged compared to those using CCR5. HS inhibition of cell attachment is thus restricted to CXCR4-using viruses (such as T-cell line-adapted HIV-1). Secondly, studies aiming at characterizing the gp120/HS complex revealed that HS binding was far more complex than previously thought: in addition to the V3 loop of CXCR4 tropic gp120, HS interacts with several other cryptic areas of the protein, which can be induced upon CD4 binding, and are conserved amongst CCR5 and CXCR4 viruses. In view of these data, this review will detail the present knowledge on HS binding to HIV-1, with regards to attachment and entry processes. It will discuss the perspective of targeting the gp120 co-receptor binding site with HS mimetic compounds, a strategy that recently gave rise to entry inhibitors that work in the low nanomolar range, independently of co-receptor usage.
Keywords: CCR5/CXCR4; HIV-1; V3 loop; attachment and entry inhibition; co-receptor binding site; glycosaminoglycan; gp120; heparan sulfate.
Figures


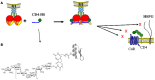
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

