Cytokinin cross-talking during biotic and abiotic stress responses
- PMID: 24312105
- PMCID: PMC3833016
- DOI: 10.3389/fpls.2013.00451
Cytokinin cross-talking during biotic and abiotic stress responses
Abstract
As sessile organisms, plants have to be able to adapt to a continuously changing environment. Plants that perceive some of these changes as stress signals activate signaling pathways to modulate their development and to enable them to survive. The complex responses to environmental cues are to a large extent mediated by plant hormones that together orchestrate the final plant response. The phytohormone cytokinin is involved in many plant developmental processes. Recently, it has been established that cytokinin plays an important role in stress responses, but does not act alone. Indeed, the hormonal control of plant development and stress adaptation is the outcome of a complex network of multiple synergistic and antagonistic interactions between various hormones. Here, we review the recent findings on the cytokinin function as part of this hormonal network. We focus on the importance of the crosstalk between cytokinin and other hormones, such as abscisic acid, jasmonate, salicylic acid, ethylene, and auxin in the modulation of plant development and stress adaptation. Finally, the impact of the current research in the biotechnological industry will be discussed.
Keywords: abscisic acid; cytokinin; hormonal crosstalk; salicylic acid; stress.
Figures
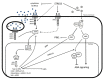
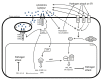
References
-
- Adie B. A. T., Pérez-Pérez J., Pérez-Pérez M. M., Godoy M., Sánchez-Serrano J.-J., Schmelz E. A., et al. (2007). ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19 1665–1681 10.1105/tpc.106.048041 - DOI - PMC - PubMed
-
- Andi S., Taguchi F., Toyoda K., Shiraishi T., Ichinose Y. (2001). Effect of methyl jasmonate on harpin-induced hypersensitive cell death, generation of hydrogen peroxide and expression of PAL mRNA in tobacco suspension cultured BY-2 cells. Plant Cell Physiol. 42 446–449 10.1093/pcp/pce056 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

