Detection of HIV-1 neutralizing antibodies in a human CD4⁺/CXCR4⁺/CCR5⁺ T-lymphoblastoid cell assay system
- PMID: 24312168
- PMCID: PMC3842913
- DOI: 10.1371/journal.pone.0077756
Detection of HIV-1 neutralizing antibodies in a human CD4⁺/CXCR4⁺/CCR5⁺ T-lymphoblastoid cell assay system
Abstract
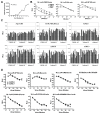
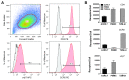
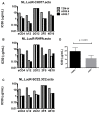
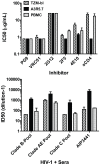
Sensitive assays are needed to meaningfully assess low levels of neutralizing antibodies (NAbs) that may be important for protection against the acquisition of HIV-1 infection in vaccine recipients. The current assay of choice uses a non-lymphoid cell line (TZM-bl) that may lack sensitivity owing to over expression of CD4 and CCR5. We used transfection of a human CD4+/CXCR4+/α4β7+ T-lymphoblastoid cell line (A3.01) with a CMV IE promoter-driven CCR5neo vector to stably express CCR5. The resulting line, designated A3R5, is permissive to a wide range of CCR5-tropic circulating strains of HIV-1, including HIV-1 molecular clones containing a Tat-inducible Renilla luciferase reporter gene and expressing multiple Env subtypes. Flow cytometric analysis found CCR5 surface expression on A3R5 cells to be markedly less than TZM-bl but similar to CD3.8 stimulated PBMC. More importantly, neutralization mediated by a diverse panel of monoclonal antibodies, HIV-1 positive polyclonal sera and sCD4 was consistently greater in A3R5 compared to TZM-bl cells. The A3R5 cell line provides a novel approach to guide the development and qualification of promising new HIV-1 vaccine immunogens.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

