Use of the γ-H2AX assay to investigate DNA repair dynamics following multiple radiation exposures
- PMID: 24312182
- PMCID: PMC3843657
- DOI: 10.1371/journal.pone.0079541
Use of the γ-H2AX assay to investigate DNA repair dynamics following multiple radiation exposures
Abstract
Radiation therapy is one of the most common and effective strategies used to treat cancer. The irradiation is usually performed with a fractionated scheme, where the dose required to kill tumour cells is given in several sessions, spaced by specific time intervals, to allow healthy tissue recovery. In this work, we examined the DNA repair dynamics of cells exposed to radiation delivered in fractions, by assessing the response of histone-2AX (H2AX) phosphorylation (γ-H2AX), a marker of DNA double strand breaks. γ-H2AX foci induction and disappearance were monitored following split dose irradiation experiments in which time interval between exposure and dose were varied. Experimental data have been coupled to an analytical theoretical model, in order to quantify key parameters involved in the foci induction process. Induction of γ-H2AX foci was found to be affected by the initial radiation exposure with a smaller number of foci induced by subsequent exposures. This was compared to chromatin relaxation and cell survival. The time needed for full recovery of γ-H2AX foci induction was quantified (12 hours) and the 1:1 relationship between radiation induced DNA double strand breaks and foci numbers was critically assessed in the multiple irradiation scenarios.
Conflict of interest statement
Figures


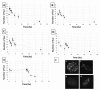
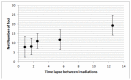




References
-
- Kavanagh J, Redmond K, Schettino G, Prise K (2013) DSB Repair - A radiation perspective. Antioxid Redox Signal [Epub ahead of print]. - PubMed
-
- Alloni D, Campa A, Friedland W, Mariotti L, Ottolenghi A (2013) Integration of Monte Carlo Simulations with PFGE Experimental Data Yields Constant RBE of 2.3 for DNA Double-Strand Break Induction by Nitrogen Ions between 125 and 225 keV/μm LET. Radiat Res 179: 690-697. doi:10.1667/R3043.1. PubMed: 23647004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

