High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena
- PMID: 24312209
- PMCID: PMC3843655
- DOI: 10.1371/journal.pone.0080302
High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena
Abstract
Background: In pulmonary tuberculosis (PTB), morbidity during treatment and residual pulmonary disability can be under-estimated.
Methods: Among adults with smear-positive PTB at an outpatient clinic in Papua, Indonesia, we assessed morbidity at baseline and during treatment, and 6-month residual disability, by measuring functional capacity (six-minute walk test [6MWT] and pulmonary function), quality of life (St George's Respiratory Questionnaire [SGRQ]) and Adverse Events ([AE]: new symptoms not present at outset). Results were compared with findings in locally-recruited volunteers.
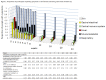
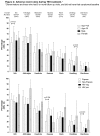
Results: 200 PTB patients and 40 volunteers were enrolled. 6WMT was 497m (interquartile range 460-529) in controls versus 408m (IQR 346-450) in PTB patients at baseline (p<0.0001) and 470m (IQR 418-515) in PTB patients after 6 months (p=0.02 versus controls). SGRQ total score was 0 units (IQR 0-2.9) in controls, versus 36.9 (27.4-52.8) in PTB patients at baseline (p<0.0001) and 4.3 (1.7-8.8) by 6 months (p<0.0001). Mean percentage of predicted FEV1 was 92% (standard deviation 19.9) in controls, versus 63% (19.4) in PTB patients at baseline (p<0.0001) and 71% (17.5) by 6 months (p<0.0001). After 6 months, 27% of TB patients still had at least moderate-severe pulmonary function impairment, and 57% still had respiratory symptoms, despite most achieving 'successful' treatment outcomes, and reporting good quality of life. More-advanced disease at baseline (longer illness duration, worse baseline X-ray) and HIV positivity predicted residual disability. AE at any time during treatment were common: itch 59%, arthralgia 58%, headache 40%, nausea 33%, vomiting 16%.
Conclusion: We found high 6-month residual pulmonary disability and high AE rates. Although PTB treatment is highly successful, the extent of morbidity during treatment and residual impairment could be overlooked if not specifically sought. Calculations of PTB-related burden of disease should acknowledge that TB-related morbidity does not stop at 6 months. Early case detection and treatment are key in minimising residual impairment.
Conflict of interest statement
Figures


References
-
- World Health Organisation (2011) Methods used to estimate the burden of disease caused by TB Annex 1, global TUBERCULOSIS Report 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

