Automated cell tracking and analysis in phase-contrast videos (iTrack4U): development of Java software based on combined mean-shift processes
- PMID: 24312283
- PMCID: PMC3842324
- DOI: 10.1371/journal.pone.0081266
Automated cell tracking and analysis in phase-contrast videos (iTrack4U): development of Java software based on combined mean-shift processes
Abstract
Cell migration is a key biological process with a role in both physiological and pathological conditions. Locomotion of cells during embryonic development is essential for their correct positioning in the organism; immune cells have to migrate and circulate in response to injury. Failure of cells to migrate or an inappropriate acquisition of migratory capacities can result in severe defects such as altered pigmentation, skull and limb abnormalities during development, and defective wound repair, immunosuppression or tumor dissemination. The ability to accurately analyze and quantify cell migration is important for our understanding of development, homeostasis and disease. In vitro cell tracking experiments, using primary or established cell cultures, are often used to study migration as cells can quickly and easily be genetically or chemically manipulated. Images of the cells are acquired at regular time intervals over several hours using microscopes equipped with CCD camera. The locations (x,y,t) of each cell on the recorded sequence of frames then need to be tracked. Manual computer-assisted tracking is the traditional method for analyzing the migratory behavior of cells. However, this processing is extremely tedious and time-consuming. Most existing tracking algorithms require experience in programming languages that are unfamiliar to most biologists. We therefore developed an automated cell tracking program, written in Java, which uses a mean-shift algorithm and ImageJ as a library. iTrack4U is a user-friendly software. Compared to manual tracking, it saves considerable amount of time to generate and analyze the variables characterizing cell migration, since they are automatically computed with iTrack4U. Another major interest of iTrack4U is the standardization and the lack of inter-experimenter differences. Finally, iTrack4U is adapted for phase contrast and fluorescent cells.
Conflict of interest statement
Figures
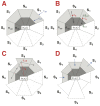

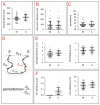
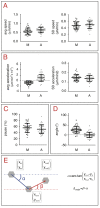
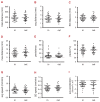
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

