Anti-cancer activity of an osthole derivative, NBM-T-BMX-OS01: targeting vascular endothelial growth factor receptor signaling and angiogenesis
- PMID: 24312323
- PMCID: PMC3842266
- DOI: 10.1371/journal.pone.0081592
Anti-cancer activity of an osthole derivative, NBM-T-BMX-OS01: targeting vascular endothelial growth factor receptor signaling and angiogenesis
Abstract
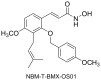
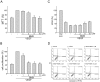
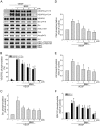
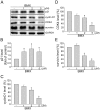
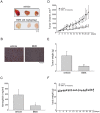
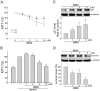
Angiogenesis occurs during tissue growth, development and wound healing. It is also required for tumor progression and represents a rational target for therapeutic intervention. NBM-T-BMX-OS01 (BMX), derived from the semisynthesis of osthole, an active ingredient isolated from Chinese herb Cnidium monnieri (L.) Cuss., was recently shown to enhance learning and memory in rats. In this study, we characterized the anti-angiogenic activities of NBM-T-BMX-OS01 (BMX) in an effort to develop novel inhibitors to suppress angiogenesis and tumor growth. BMX inhibited vascular endothelial growth factor (VEGF)-induced proliferation, migration and endothelial tube formation in human umbilical endothelial cells (HUVECs). BMX also attenuated VEGF-induced microvessel sprouting from aortic rings ex vivo and reduced HCT116 colorectal cancer cells-induced angiogenesis in vivo. Moreover, BMX inhibited the phosphorylation of VEGFR2, FAK, Akt and ERK in HUVECs exposed to VEGF. BMX was also shown to inhibit HCT116 cell proliferation and to suppress the growth of subcutaneous xenografts of HCT116 cells in vivo. Taken together, this study provides evidence that BMX modulates vascular endothelial cell remodeling and leads to the inhibition of tumor angiogenesis. These results also support the role of BMX as a potential drug candidate and warrant the clinical development in the treatment of cancer.
Conflict of interest statement
Figures

Similar articles
-
Antiangiogenic mechanisms of PJ-8, a novel inhibitor of vascular endothelial growth factor receptor signaling.Carcinogenesis. 2012 May;33(5):1022-30. doi: 10.1093/carcin/bgs127. Epub 2012 Mar 20. Carcinogenesis. 2012. PMID: 22436611
-
NBM-T-BMX-OS01, an Osthole Derivative, Sensitizes Human Lung Cancer A549 Cells to Cisplatin through AMPK-Dependent Inhibition of ERK and Akt Pathway.Cell Physiol Biochem. 2015;36(3):893-906. doi: 10.1159/000430264. Epub 2015 Jun 9. Cell Physiol Biochem. 2015. PMID: 26065336
-
PPemd26, an anthraquinone derivative, suppresses angiogenesis via inhibiting VEGFR2 signalling.Br J Pharmacol. 2014 Dec;171(24):5728-42. doi: 10.1111/bph.12872. Br J Pharmacol. 2014. PMID: 25091695 Free PMC article.
-
VEGF targets the tumour cell.Nat Rev Cancer. 2013 Dec;13(12):871-82. doi: 10.1038/nrc3627. Nat Rev Cancer. 2013. PMID: 24263190 Free PMC article. Review.
-
Anticancer potential of osthole: targeting gynecological tumors and breast cancer.Pharmacol Rep. 2025 Feb;77(1):87-102. doi: 10.1007/s43440-024-00685-3. Epub 2024 Dec 2. Pharmacol Rep. 2025. PMID: 39617816 Review.
Cited by
-
Osthole: A Coumarin with Dual Roles in Biology and Chemistry.Biology (Basel). 2025 May 22;14(6):588. doi: 10.3390/biology14060588. Biology (Basel). 2025. PMID: 40563840 Free PMC article. Review.
-
Phytochemistry, Ethnopharmacology, Pharmacokinetics and Toxicology of Cnidium monnieri (L.) Cusson.Int J Mol Sci. 2020 Feb 3;21(3):1006. doi: 10.3390/ijms21031006. Int J Mol Sci. 2020. PMID: 32028721 Free PMC article. Review.
-
Osthole sensitizes with radiotherapy to suppress tumorigenesis of human nasopharyngeal carcinoma in vitro and in vivo.Cancer Manag Res. 2018 Nov 8;10:5471-5477. doi: 10.2147/CMAR.S182798. eCollection 2018. Cancer Manag Res. 2018. Retraction in: Cancer Manag Res. 2023 Mar 24;15:319-320. doi: 10.2147/CMAR.S412641. PMID: 30519095 Free PMC article. Retracted.
-
Plant metabolites and functional foods in metastatic breast cancer: a supportive strategy for management.Front Pharmacol. 2025 Jul 23;16:1631232. doi: 10.3389/fphar.2025.1631232. eCollection 2025. Front Pharmacol. 2025. PMID: 40771913 Free PMC article. Review.
-
BMX, a specific HDAC8 inhibitor, with TMZ for advanced CRC therapy: a novel synergic effect to elicit p53-, β-catenin- and MGMT-dependent apoptotic cell death.Cell Commun Signal. 2022 Dec 27;20(1):200. doi: 10.1186/s12964-022-01007-x. Cell Commun Signal. 2022. PMID: 36575468 Free PMC article.
References
-
- Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249–257. - PubMed
-
- Dvorak HF (2005) Angiogenesis: update 2005. J Thromb Haemost 3: 1835–1842. - PubMed
-
- Thairu N, Kiriakidis S, Dawson P, Paleolog E (2011) Angiogenesis as a therapeutic target in arthritis in 2011: learning the lessons of the colorectal cancer experience. Angiogenesis 14: 223–234. - PubMed
-
- Cooney MM, van Heeckeren W, Bhakta S, Ortiz J, Remick SC (2006) Drug insight: vascular disrupting agents and angiogenesis–novel approaches for drug delivery. Nat Clin Pract Oncol 3: 682–692. - PubMed
-
- Won MS, Im N, Park S, Boovanahalli SK, Jin Y, et al. (2009) A novel benzimidazole analogue inhibits the hypoxia-inducible factor (HIF)-1 pathway. Biochem Biophys Res Commun 385: 16–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

