Smooth muscle LDL receptor-related protein-1 deletion induces aortic insufficiency and promotes vascular cardiomyopathy in mice
- PMID: 24312398
- PMCID: PMC3843717
- DOI: 10.1371/journal.pone.0082026
Smooth muscle LDL receptor-related protein-1 deletion induces aortic insufficiency and promotes vascular cardiomyopathy in mice
Abstract
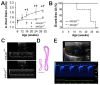
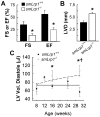
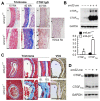
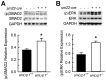
Valvular disease is common in patients with Marfan syndrome and can lead to cardiomyopathy. However, some patients develop cardiomyopathy in the absence of hemodynamically significant valve dysfunction, suggesting alternative mechanisms of disease progression. Disruption of LDL receptor-related protein-1 (Lrp1) in smooth muscle cells has been shown to cause vascular pathologies similar to Marfan syndrome, with activation of smooth muscle cells, vascular dysfunction and aortic aneurysms. This study used echocardiography and blood pressure monitoring in mouse models to determine whether inactivation of Lrp1 in vascular smooth muscle leads to cardiomyopathy, and if so, whether the mechanism is a consequence of valvular disease. Hemodynamic changes during treatment with captopril were also assessed. Dilation of aortic roots was observed in young Lrp1-knockout mice and progressed as they aged, whereas no significant aortic dilation was detected in wild type littermates. Diastolic blood pressure was lower and pulse pressure higher in Lrp1-knockout mice, which was normalized by treatment with captopril. Aortic dilation was followed by development of aortic insufficiency and subsequent dilated cardiomyopathy due to valvular disease. Thus, smooth muscle cell Lrp1 deficiency results in aortic dilation and insufficiency that causes secondary cardiomyopathy that can be improved by captopril. These findings provide novel insights into mechanisms of cardiomyopathy associated with vascular activation and offer a new model of valvular cardiomyopathy.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

