Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies
- PMID: 24312451
- PMCID: PMC3849193
- DOI: 10.1371/journal.pone.0079804
Central role for MCP-1/CCL2 in injury-induced inflammation revealed by in vitro, in silico, and clinical studies
Abstract
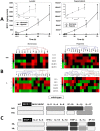
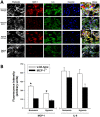
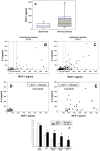
The translation of in vitro findings to clinical outcomes is often elusive. Trauma/hemorrhagic shock (T/HS) results in hepatic hypoxia that drives inflammation. We hypothesize that in silico methods would help bridge in vitro hepatocyte data and clinical T/HS, in which the liver is a primary site of inflammation. Primary mouse hepatocytes were cultured under hypoxia (1% O2) or normoxia (21% O2) for 1-72 h, and both the cell supernatants and protein lysates were assayed for 18 inflammatory mediators by Luminex™ technology. Statistical analysis and data-driven modeling were employed to characterize the main components of the cellular response. Statistical analyses, hierarchical and k-means clustering, Principal Component Analysis, and Dynamic Network Analysis suggested MCP-1/CCL2 and IL-1α as central coordinators of hepatocyte-mediated inflammation in C57BL/6 mouse hepatocytes. Hepatocytes from MCP-1-null mice had altered dynamic inflammatory networks. Circulating MCP-1 levels segregated human T/HS survivors from non-survivors. Furthermore, T/HS survivors with elevated early levels of plasma MCP-1 post-injury had longer total lengths of stay, longer intensive care unit lengths of stay, and prolonged requirement for mechanical ventilation vs. those with low plasma MCP-1. This study identifies MCP-1 as a main driver of the response of hepatocytes in vitro and as a biomarker for clinical outcomes in T/HS, and suggests an experimental and computational framework for discovery of novel clinical biomarkers in inflammatory diseases.
Conflict of interest statement
Figures





References
-
- Malarkey DE, Johnson K, Ryan L, Boorman G, Maronpot RR (2005) New insights into functional aspects of liver morphology. Toxicol Pathol 33: 27–34. - PubMed
-
- McCloskey CA, Kameneva MV, Uryash A, Gallo DJ, Billiar TR (2004) Tissue hypoxia activates JNK in the liver during hemorrhagic shock. Shock 22: 380–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

