A balanced IL-1β activity is required for host response to Citrobacter rodentium infection
- PMID: 24312491
- PMCID: PMC3846666
- DOI: 10.1371/journal.pone.0080656
A balanced IL-1β activity is required for host response to Citrobacter rodentium infection
Abstract
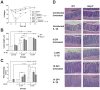
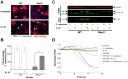
Microbial sensing plays essential roles in the innate immune response to pathogens. In particular, NLRP3 forms a multiprotein inflammasome complex responsible for the maturation of interleukin (IL)-1β. Our aim was to delineate the role of the NLRP3 inflammasome in macrophages, and the contribution of IL-1β to the host defense against Citrobacter rodentium acute infection in mice. Nlrp3(-/-) and background C57BL/6 (WT) mice were infected by orogastric gavage, received IL-1β (0.5 µg/mouse; ip) on 0, 2, and 4 days post-infection (DPI), and assessed on 6 and 10 DPI. Infected Nlrp3(-/-) mice developed severe colitis; IL-1β treatments reduced colonization, abrogated dissemination of bacteria to mesenteric lymph nodes, and protected epithelial integrity of infected Nlrp3(-/-) mice. In contrast, IL-1β treatments of WT mice had an opposite effect with increased penetration of bacteria and barrier disruption. Microscopy showed reduced damage in Nlrp3(-/-) mice, and increased severity of disease in WT mice with IL-1β treatments, in particular on 10 DPI. Secretion of some pro-inflammatory plasma cytokines was dissipated in Nlrp3(-/-) compared to WT mice. IL-1β treatments elevated macrophage infiltration into infected crypts in Nlrp3(-/-) mice, suggesting that IL-1β may improve macrophage function, as exogenous administration of IL-1β increased phagocytosis of C. rodentium by peritoneal Nlrp3(-/-) macrophages in vitro. As well, the exogenous administration of IL-1β to WT peritoneal macrophages damaged the epithelial barrier of C. rodentium-infected polarized CMT-93 cells. Treatment of Nlrp3(-/-) mice with IL-1β seems to confer protection against C. rodentium infection by reducing colonization, protecting epithelial integrity, and improving macrophage activity, while extraneous IL-1β appeared to be detrimental to WT mice. Together, these findings highlight the importance of balanced cytokine responses as IL-1β improved bacterial clearance in Nlrp3(-/-) mice but increased tissue damage when given to WT mice.
Conflict of interest statement
Figures




References
-
- Borenshtein D, McBee ME, Schauer DB (2008) Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol 24: 32–37. - PubMed
-
- Wiles S, Dougan G, Frankel G (2005) Emergence of a ‘hyperinfectious’ bacterial state after passage of Citrobacter rodentium through the host gastrointestinal tract. Cell Microbiol 7: 1163–1172. - PubMed
-
- Wiles S, Clare S, Harker J, Huett A, Young D, et al. (2004) Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium . Cell Microbiol 6: 963–972. - PubMed
-
- MacDonald TT, Frankel G, Dougan G, Goncalves NS, Simmons C (2003) Host defences to Citrobacter rodentium . Int J Med Microbiol 293: 87–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

