PRICKLE1 interaction with SYNAPSIN I reveals a role in autism spectrum disorders
- PMID: 24312498
- PMCID: PMC3849077
- DOI: 10.1371/journal.pone.0080737
PRICKLE1 interaction with SYNAPSIN I reveals a role in autism spectrum disorders
Abstract
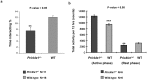
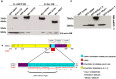
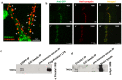
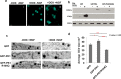
The frequent comorbidity of Autism Spectrum Disorders (ASDs) with epilepsy suggests a shared underlying genetic susceptibility; several genes, when mutated, can contribute to both disorders. Recently, PRICKLE1 missense mutations were found to segregate with ASD. However, the mechanism by which mutations in this gene might contribute to ASD is unknown. To elucidate the role of PRICKLE1 in ASDs, we carried out studies in Prickle1(+/-) mice and Drosophila, yeast, and neuronal cell lines. We show that mice with Prickle1 mutations exhibit ASD-like behaviors. To find proteins that interact with PRICKLE1 in the central nervous system, we performed a yeast two-hybrid screen with a human brain cDNA library and isolated a peptide with homology to SYNAPSIN I (SYN1), a protein involved in synaptogenesis, synaptic vesicle formation, and regulation of neurotransmitter release. Endogenous Prickle1 and Syn1 co-localize in neurons and physically interact via the SYN1 region mutated in ASD and epilepsy. Finally, a mutation in PRICKLE1 disrupts its ability to increase the size of dense-core vesicles in PC12 cells. Taken together, these findings suggest PRICKLE1 mutations contribute to ASD by disrupting the interaction with SYN1 and regulation of synaptic vesicles.
Conflict of interest statement
Figures




References
-
- Lord C, Cook EH, Leventhal BL, Amaral DG (2000) Autism spectrum disorders. Neuron 28: 355–363. - PubMed
-
- Bailey A, Phillips W, Rutter M (1996) Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry 37: 89–126. - PubMed
-
- Dravet C (2002) [The behavioral disorders in epilepsy]. Revue neurologique 158: 4S33–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

