Pre-clinical development of a recombinant, replication-competent adenovirus serotype 4 vector vaccine expressing HIV-1 envelope 1086 clade C
- PMID: 24312658
- PMCID: PMC3849430
- DOI: 10.1371/journal.pone.0082380
Pre-clinical development of a recombinant, replication-competent adenovirus serotype 4 vector vaccine expressing HIV-1 envelope 1086 clade C
Abstract
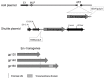
Background: There is a well-acknowledged need for an effective AIDS vaccine that protects against HIV-1 infection or limits in vivo viral replication. The objective of these studies is to develop a replication-competent, vaccine vector based on the adenovirus serotype 4 (Ad4) virus expressing HIV-1 envelope (Env) 1086 clade C glycoprotein. Ad4 recombinant vectors expressing Env gp160 (Ad4Env160), Env gp140 (Ad4Env140), and Env gp120 (Ad4Env120) were evaluated.
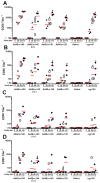
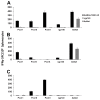
Methods: The recombinant Ad4 vectors were generated with a full deletion of the E3 region of Ad4 to accommodate the env gene sequences. The vaccine candidates were assessed in vitro following infection of A549 cells for Env-specific protein expression and for posttranslational transport to the cell surface as monitored by the binding of broadly neutralizing antibodies (bNAbs). The capacity of the Ad4Env vaccines to induce humoral immunity was evaluated in rabbits for Env gp140 and V1V2-specific binding antibodies, and HIV-1 pseudovirus neutralization. Mice immunized with the Ad4Env160 vaccine were assessed for IFNγ T cell responses specific for overlapping Env peptide sets.
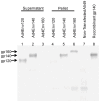
Results: Robust Env protein expression was confirmed by western blot analysis and recognition of cell surface Env gp160 by multiple bNAbs. Ad4Env vaccines induced humoral immune responses in rabbits that recognized Env 1086 gp140 and V1V2 polypeptide sequences derived from 1086 clade C, A244 clade AE, and gp70 V1V2 CASE A2 clade B fusion protein. The immune sera efficiently neutralized tier 1 clade C pseudovirus MW965.26 and neutralized the homologous and heterologous tier 2 pseudoviruses to a lesser extent. Env-specific T cell responses were also induced in mice following Ad4Env160 vector immunization.
Conclusions: The Ad4Env vaccine vectors express high levels of Env glycoprotein and induce both Env-specific humoral and cellular immunity thus supporting further development of this new Ad4 HIV-1 Env vaccine platform in Phase 1 clinical trials.
Conflict of interest statement
Figures

References
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R et al. (2008) Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881-1893. doi:10.1016/S0140-6736(08)61591-3. PubMed: 19012954. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

