Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans
- PMID: 24312900
- PMCID: PMC3832793
- DOI: 10.3389/fcimb.2013.00080
Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans
Abstract
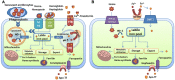
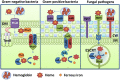
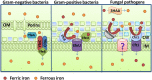
Iron is the most abundant transition metal in the human body and its bioavailability is stringently controlled. In particular, iron is tightly bound to host proteins such as transferrin to maintain homeostasis, to limit potential damage caused by iron toxicity under physiological conditions and to restrict access by pathogens. Therefore, iron acquisition during infection of a human host is a challenge that must be surmounted by every successful pathogenic microorganism. Iron is essential for bacterial and fungal physiological processes such as DNA replication, transcription, metabolism, and energy generation via respiration. Hence, pathogenic bacteria and fungi have developed sophisticated strategies to gain access to iron from host sources. Indeed, siderophore production and transport, iron acquisition from heme and host iron-containing proteins such as hemoglobin and transferrin, and reduction of ferric to ferrous iron with subsequent transport are all strategies found in bacterial and fungal pathogens of humans. This review focuses on a comparison of these strategies between bacterial and fungal pathogens in the context of virulence and the iron limitation that occurs in the human body as a mechanism of innate nutritional defense.
Keywords: heme; hemoglobin; iron; microbial pathogenesis; siderophores; transferrin.
Figures


References
-
- Ahluwalia M., Brummer E., Sridhar S., Singh R., Stevens D. A. (2001). Isolation and characterisation of an anticryptococcal protein in human cerebrospinal fluid. J. Med. Microbiol. 50, 83–89 - PubMed
-
- Aisen P., Brown E. B. (1977). The iron-binding function of transferrin in iron metabolism. Semin. Hematol. 14, 31–53 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

