Radical [3 + 2]-annulation of divinylcyclopropanes: rapid synthesis of complex meloscine analogs
- PMID: 24313360
- PMCID: PMC3910537
- DOI: 10.1021/ol403078e
Radical [3 + 2]-annulation of divinylcyclopropanes: rapid synthesis of complex meloscine analogs
Abstract
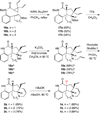
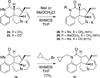
A radical [3 + 2]-divinylcyclopropane annulation cascade has been extended to encompass five D-ring variants of the meloscine/epimeloscine core structure. Representative ABCD tetracyclic intermediates were further elaborated with novel substituted E-rings through subsequent transformations of advanced intermediates that provided opportunities for late-stage variation of the B-ring (lactam) N-substituents which were also developed.
Figures



Similar articles
-
A short total synthesis of (±)-epimeloscine and (±)-meloscine enabled by a cascade radical annulation of a divinylcyclopropane.J Am Chem Soc. 2011 Jul 13;133(27):10376-8. doi: 10.1021/ja2042854. Epub 2011 Jun 16. J Am Chem Soc. 2011. PMID: 21663316 Free PMC article.
-
Allenyl azide cycloaddition chemistry: application to the total synthesis of (±)-meloscine.Org Lett. 2012 Feb 3;14(3):934-7. doi: 10.1021/ol203463n. Epub 2012 Jan 13. Org Lett. 2012. PMID: 22242696 Free PMC article.
-
Total synthesis of meloscine by a [2+2]-photocycloaddition/ring-expansion route.Chemistry. 2009;15(14):3509-25. doi: 10.1002/chem.200802383. Chemistry. 2009. PMID: 19219879
-
[Synthetic Study of Polycyclic Natural Products Based on Development of New Strategy].Yakugaku Zasshi. 2021;141(8):985-994. doi: 10.1248/yakushi.21-00054. Yakugaku Zasshi. 2021. PMID: 34334550 Review. Japanese.
-
Morita-Baylis-Hillman adduct derivatives (MBHADs): versatile reactivity in Lewis base-promoted annulation.Org Biomol Chem. 2015 Aug 28;13(32):8578-95. doi: 10.1039/c5ob00865d. Epub 2015 Jul 2. Org Biomol Chem. 2015. PMID: 26133693 Review.
Cited by
-
Synthesis of (±)-tetrapetalone A-Me aglycon.Angew Chem Int Ed Engl. 2014 Aug 25;53(35):9334-8. doi: 10.1002/anie.201404410. Epub 2014 Jul 7. Angew Chem Int Ed Engl. 2014. PMID: 25045072 Free PMC article.
-
Rearrangement reactions of 1,1-divinyl-2-phenylcyclopropanes.J Am Chem Soc. 2015 Jan 14;137(1):322-7. doi: 10.1021/ja510608u. Epub 2014 Dec 22. J Am Chem Soc. 2015. PMID: 25530073 Free PMC article.
-
Highly functionalized donor-acceptor cyclopropanes applied toward the synthesis of the Melodinus alkaloids.Tetrahedron Lett. 2015 Jun 3;56(23):2983-2990. doi: 10.1016/j.tetlet.2014.09.016. Tetrahedron Lett. 2015. PMID: 26120207 Free PMC article.
References
-
- Clemons PA, Wilson JA, Dančik V, Muller S, Carrinski HA, Wagner BK, Koehler AN, Schreiber SL. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6817–6822. - PMC - PubMed
- Clemons PA, Bodycombe NE, Carrinski HA, Wilson JA, Shamji AF, Wagner BK, Koehler AN, Schreiber SL. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18787–18792. - PMC - PubMed
- Lovering F, Bikker J, Humblet C. J. Med. Chem. 2009;52:6752–6756. - PubMed
- Camp D, Davis RA, Campitelli M, Ebdon J, Quinn RJ. J. Nat. Prod. 2012;75:72–81. - PubMed
-
-
(a) Isolation: Bernauer K, Englert G, Vetter W, Weiss E. Helv. Chim. Acta. 1969;52:1886–1905. (b) Short review: Szabó LF. Arkivoc. 2007;7:280–290.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

