Radical [3 + 2]-annulation of divinylcyclopropanes: rapid synthesis of complex meloscine analogs
- PMID: 24313360
- PMCID: PMC3910537
- DOI: 10.1021/ol403078e
Radical [3 + 2]-annulation of divinylcyclopropanes: rapid synthesis of complex meloscine analogs
Abstract
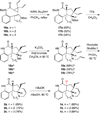
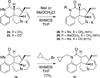
A radical [3 + 2]-divinylcyclopropane annulation cascade has been extended to encompass five D-ring variants of the meloscine/epimeloscine core structure. Representative ABCD tetracyclic intermediates were further elaborated with novel substituted E-rings through subsequent transformations of advanced intermediates that provided opportunities for late-stage variation of the B-ring (lactam) N-substituents which were also developed.
Figures
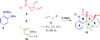
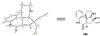



References
-
- Clemons PA, Wilson JA, Dančik V, Muller S, Carrinski HA, Wagner BK, Koehler AN, Schreiber SL. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6817–6822. - PMC - PubMed
- Clemons PA, Bodycombe NE, Carrinski HA, Wilson JA, Shamji AF, Wagner BK, Koehler AN, Schreiber SL. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18787–18792. - PMC - PubMed
- Lovering F, Bikker J, Humblet C. J. Med. Chem. 2009;52:6752–6756. - PubMed
- Camp D, Davis RA, Campitelli M, Ebdon J, Quinn RJ. J. Nat. Prod. 2012;75:72–81. - PubMed
-
-
(a) Isolation: Bernauer K, Englert G, Vetter W, Weiss E. Helv. Chim. Acta. 1969;52:1886–1905. (b) Short review: Szabó LF. Arkivoc. 2007;7:280–290.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

