Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus
- PMID: 24313680
- PMCID: PMC4394906
- DOI: 10.1111/ejn.12450
Re-evaluation of connexins associated with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus
Abstract
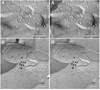
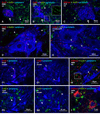
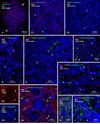
Electrical synapses formed by neuronal gap junctions composed of connexin36 (Cx36) are a common feature in mammalian brain circuitry, but less is known about their deployment in spinal cord. It has been reported based on connexin mRNA and/or protein detection that developing and/or mature motoneurons express a variety of connexins, including Cx26, Cx32, Cx36 and Cx43 in trigeminal motoneurons, Cx36, Cx37, Cx40, Cx43 and Cx45 in spinal motoneurons, and Cx32 in sexually dimorphic motoneurons. We re-examined the localization of these connexins during postnatal development and in adult rat and mouse using immunofluorescence labeling for each connexin. We found Cx26 in association only with leptomeninges in the trigeminal motor nucleus (Mo5), Cx32 only with oligodendrocytes and myelinated fibers among motoneurons in this nucleus and in the spinal cord, and Cx37, Cx40 and Cx45 only with blood vessels in the ventral horn of spinal cord, including those among motoneurons. By freeze-fracture replica immunolabeling, > 100 astrocyte gap junctions but no neuronal gap junctions were found based on immunogold labeling for Cx43, whereas 16 neuronal gap junctions at postnatal day (P)4, P7 and P18 were detected based on Cx36 labeling. Punctate labeling for Cx36 was localized to the somatic and dendritic surfaces of peripherin-positive motoneurons in the Mo5, motoneurons throughout the spinal cord, and sexually dimorphic motoneurons at lower lumbar levels. In studies of electrical synapses and electrical transmission between developing and between adult motoneurons, our results serve to focus attention on mediation of this transmission by gap junctions composed of Cx36.
Keywords: electrical coupling; gap junctions; sexually dimorphic motoneurons.
© 2013 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

