Airway contractility in the precision-cut lung slice after cryopreservation
- PMID: 24313705
- PMCID: PMC4068941
- DOI: 10.1165/rcmb.2013-0166MA
Airway contractility in the precision-cut lung slice after cryopreservation
Abstract
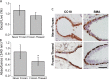
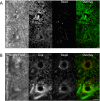
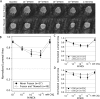
An emerging tool in airway biology is the precision-cut lung slice (PCLS). Adoption of the PCLS as a model for assessing airway reactivity has been hampered by the limited time window within which tissues remain viable. Here we demonstrate that the PCLS can be frozen, stored long-term, and then thawed for later experimental use. Compared with the never-frozen murine PCLS, the frozen-thawed PCLS shows metabolic activity that is decreased to an extent comparable to that observed in other cryopreserved tissues but shows no differences in cell viability or in airway caliber responses to the contractile agonist methacholine or the relaxing agonist chloroquine. These results indicate that freezing and long-term storage is a feasible solution to the problem of limited viability of the PCLS in culture.
Figures
References
-
- Minshall E, Wang CG, Dandurand R, Eidelman D. Heterogeneity of responsiveness of individual airways in cultured lung explants. Can J Physiol Pharmacol. 1997;75:911–916. - PubMed
-
- Crowther SD, Chapman ID, Morley J. Heterogeneity of airway hyperresponsiveness. Clin Exp Allergy. 1997;27:606–616. - PubMed
-
- Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA, Jr, Butler JP, Fredberg JJ. Selected contribution: time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol (1985) 2001;91:986–994. - PubMed
-
- King GG, Carroll JD, Müller NL, Whittall KP, Gao M, Nakano Y, Paré PD. Heterogeneity of narrowing in normal and asthmatic airways measured by HRCT. Eur Respir J. 2004;24:211–218. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

