Moxifloxacin dosing in post-bariatric surgery patients
- PMID: 24313873
- PMCID: PMC4168383
- DOI: 10.1111/bcp.12302
Moxifloxacin dosing in post-bariatric surgery patients
Abstract
Introduction: Given the ever increasing number of obese patients and obesity related bypass surgery, dosing recommendations in the post-bypass population are needed. Using a population pharmacokinetic (PK) analysis and PK-pharmacodynamic (PD) simulations, we investigated whether adequate moxifloxacin concentrations are achieved in this population.
Methods: In this modelling and simulation study we used data from a trial on moxifloxacin PK. In this trial, volunteers who had previously undergone bariatric surgery (at least 6 months prior to inclusion), received two doses (intravenous and oral) of 400 mg moxifloxacin administered on two occasions.
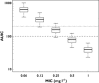
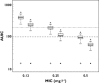
Results: In contrast to other papers, we found that moxifloxacin PK were best described by a three compartmental model using lean body mass (LBM) as a predictor for moxifloxacin clearance. Furthermore, we showed that the probability of target attainment for bacterial eradication against a hypothetical Streptococcus pneumoniae infection is compromised in patients with higher LBM, especially when targeting microorganisms with minimum inhibitory concentrations (MICs) of 0.5 mg l(-1) or higher (probability of target attainment (PTA) approaching zero). When considering the targets for suppression of bacterial resistance formation, even at MIC values as low as 0.25 mg l(-1) , standard moxifloxacin dosing does not attain adequate levels in this population. Furthermore, for patients with a LBM of 78 kg or higher, the probability of hitting this target approaches zero.
Conclusions: Throughout our PK-PD simulation study, it became apparent that, whenever optimal bacterial resistance suppression is deemed necessary, the standard moxifloxacin dosing will not be sufficient. Furthermore, our study emphasizes the need for a LBM based individualized dosing of moxifloxacin in this patient population.
Keywords: NONMEM; PK-PD; bariatric surgery; moxifloxacin; pharmacokinetics.
© 2013 The British Pharmacological Society.
Figures





References
-
- Brocks DR, Ben-Eltriki M, Gabr RQ, Padwal RS. The effects of gastric bypass surgery on drug absorption and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8:1505–1519. - PubMed
-
- Padwal RS, Ben-Eltriki M, Wang XM, Langkaas LA, Sharma AM, Birch DW, Karmali S, Brocks DR. Effect of gastric bypass surgery on azithromycin oral bioavailability. J Antimicrob Chemother. 2012;67:2203–2206. - PubMed
-
- De Smet J, Colin P, De Paepe P, Ruige J, Batens H, Van Nieuwenhove Y, Vogelaers D, Blot S, Van Bocxlaer J, Van Bortel LM, Boussery K. Oral bioavailability of moxifloxacin after Roux-en-Y gastric bypass surgery. J Antimicrob Chemother. 2012;67:226–229. - PubMed
-
- Wiemken TL, Peyrani P, Ramirez JA. Global changes in the epidemiology of community-acquired pneumonia. Semin Respir Crit Care Med. 2012;33:213–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

