The mechanisms and physiological relevance of glycocalyx degradation in hepatic ischemia/reperfusion injury
- PMID: 24313895
- PMCID: PMC4123469
- DOI: 10.1089/ars.2013.5751
The mechanisms and physiological relevance of glycocalyx degradation in hepatic ischemia/reperfusion injury
Abstract
Significance: Hepatic ischemia/reperfusion (I/R) injury is an inevitable side effect of major liver surgery that can culminate in liver failure. The bulk of I/R-induced liver injury results from an overproduction of reactive oxygen and nitrogen species (ROS/RNS), which inflict both parenchymal and microcirculatory damage. A structure that is particularly prone to oxidative attack and modification is the glycocalyx (GCX), a meshwork of proteoglycans and glycosaminoglycans (GAGs) that covers the lumenal endothelial surface and safeguards microvascular homeostasis. ROS/RNS-mediated degradation of the GCX may exacerbate I/R injury by, for example, inducing vasoconstriction, facilitating leukocyte adherence, and directly activating innate immune cells.
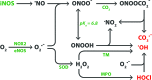
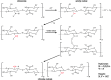
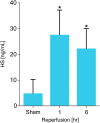
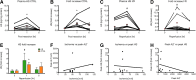
Recent advances: Preliminary experiments revealed that hepatic sinusoids contain a functional GCX that is damaged during murine hepatic I/R and major liver surgery in patients. There are three ROS that mediate GCX degradation: hydroxyl radicals, carbonate radical anions, and hypochlorous acid (HOCl). HOCl converts GAGs in the GCX to GAG chloramides that become site-specific targets for oxidizing and reducing species and are more efficiently fragmented than the parent molecules. In addition to ROS/RNS, the GAG-degrading enzyme heparanase acts at the endothelial surface to shed the GCX.
Critical issues: The GCX seems to be degraded during major liver surgery, but the underlying cause remains ill-defined.
Future directions: The relative contribution of the different ROS and RNS intermediates to GCX degradation in vivo, the immunogenic potential of the shed GCX fragments, and the role of heparanase in liver I/R injury all warrant further investigation.
Figures


References
-
- Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, and Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325, 2004 - PubMed
-
- Akeel A, Sibanda S, Martin SW, Paterson AW, and Parsons BJ. Chlorination and oxidation of heparin and hyaluronan by hypochlorous acid and hypochlorite anions: effect of sulfate groups on reaction pathways and kinetics. Free Radic Biol Med 56: 72–88, 2013 - PubMed
-
- Al-Assaf S, Navaratnam S, Parsons BJ, and Phillips GO. Chain scission of hyaluronan by peroxynitrite. Arch Biochem Biophys 411: 73–82, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

