Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise
- PMID: 24313936
- PMCID: PMC4029611
- DOI: 10.1186/1743-7075-10-69
Supplemental vitamin D enhances the recovery in peak isometric force shortly after intense exercise
Abstract
Background: Serum 25-hydroxyvitamin D (25(OH)D) concentrations associate with skeletal muscle weakness (i.e., deficit in skeletal muscle strength) after muscular injury or damage. Although supplemental vitamin D increases serum 25(OH)D concentrations, it is unknown if supplemental vitamin D enhances strength recovery after a damaging event.
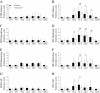
Methods: Reportedly healthy and modestly active (30 minute of continuous physical activity at least 3 time/week) adult males were randomly assigned to a placebo (n = 13, age, 31(5) y; BMI, 26.9(4.2) kg/m2; serum 25(OH)D, 31.0(8.2) ng/mL) or vitamin D (cholecalciferol, 4000 IU; n = 15; age, 30(6) y; BMI, 27.6(6.0) kg/m2; serum 25(OH)D, 30.5(9.4) ng/mL) supplement. Supplements were taken daily for 35-d. After 28-d of supplementation, one randomly selected leg performed an exercise protocol (10 sets of 10 repetitive eccentric-concentric jumps on a custom horizontal plyo-press at 75% of body mass with a 20 second rest between sets) intended to induce muscle damage. During the exercise protocol, subjects were allowed to perform presses if they were unable to complete two successive jumps. Circulating chemistries (25(OH)D and alanine (ALT) and aspartate (AST) aminotransferases), single-leg peak isometric force, and muscle soreness were measured before supplementation. Circulating chemistries, single-leg peak isometric force, and muscle soreness were also measured before (immediately) and after (immediately, 1-h [blood draw only], 24-h, 48-h, 72-h, and 168-h) the damaging event.
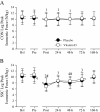
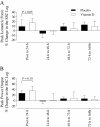
Results: Supplemental vitamin D increased serum 25(OH)D concentrations (P < 0.05; ≈70%) and enhanced the recovery in peak isometric force after the damaging event (P < 0.05; ≈8% at 24-h). Supplemental vitamin D attenuated (P < 0.05) the immediate and delayed (48-h, 72-h, or 168-h) increase in circulating biomarkers representative of muscle damage (ALT or AST) without ameliorating muscle soreness (P > 0.05).
Conclusions: We conclude that supplemental vitamin D may serve as an attractive complementary approach to enhance the recovery of skeletal muscle strength following intense exercise in reportedly active adults with a sufficient vitamin D status prior to supplementation.
Figures




References
-
- Floyd M, Ayyar DR, Barwick DD, Hudgson P, Weightman D. Myopathy in chronic renal failure. Q J Med. 1974;10:509–524. - PubMed
-
- de Boland AR, Boland R. In vitro cellular muscle calcium metabolism. Characterization of effects of 1,25-dihydroxy-vitamin D3 and 25-hydroxy-vitamin D3. Z Naturforsch C. 1985;10:102–108. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

