Phenotype and functionality of CD4+ and CD8+ T cells in the upper reproductive tract of healthy premenopausal women
- PMID: 24313954
- PMCID: PMC3947236
- DOI: 10.1111/aji.12182
Phenotype and functionality of CD4+ and CD8+ T cells in the upper reproductive tract of healthy premenopausal women
Abstract
Problem: The goal of this study was to investigate the phenotype and functional responsiveness of CD4(+) and CD8(+) T-cells in the upper reproductive tract of healthy premenopausal women. The lower reproductive tract is frequently studied as a site of sexually transmitted infections; however, the upper reproductive tract may also be a portal of entry and dissemination for pathogens, including HIV-1.
Method of study: Endometrial biopsy, endocervical curettage, cytobrush, and blood were collected during mid-luteal phase from 23 healthy women. T-cells were isolated and analyzed by flow cytometry.
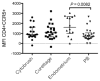
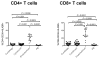
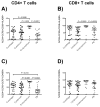
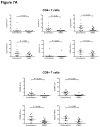
Results: As compared with their counterparts in blood, endometrial and endocervical T-cells had enhanced CCR5 expression, and were enriched for activated, effector memory cells. Endometrial T-cells were more responsive to polyclonal stimuli, producing a broad range of cytokines and chemokines.
Conclusion: These findings underscore the responsiveness of endometrial T-cells to stimulation, and reveal their activated phenotype. These findings also suggest susceptibility of the upper reproductive tract to HIV-1 infection.
Keywords: CTL; HIV; STD; T-cell; endocervix; endometrium.
© 2013 John Wiley & Sons Ltd.
Figures




References
-
- Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstetrics and gynecology. 2000;96:431–439. - PubMed
-
- Norvell MK, Benrubi GI, Thompson RJ. Investigation of microtrauma after sexual intercourse. The Journal of reproductive medicine. 1984;29:269–271. - PubMed
-
- Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. - PubMed
-
- Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

