Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid
- PMID: 24313976
- PMCID: PMC4028745
- DOI: 10.1186/1423-0127-20-90
Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid
Abstract
Background: The roles of caspase 3 on the kainic acid-mediated neurodegeneration, dendritic plasticity alteration, neurogenesis, microglial activation and gliosis are not fully understood. Here, we investigate hippocampal changes using a mouse model that receive a single kainic acid-intracerebral ventricle injection. The effects of caspase 3 inhibition on these changes were detected during a period of 1 to 7 days post kainic acid injection.
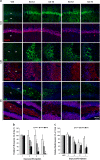
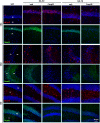
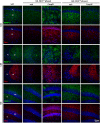
Result: Neurodegeneration was assessed by Fluoro-Jade B staining and neuronal nuclei protein (NeuN) immunostaining. Neurogenesis, gliosis, neuritic plasticity alteration and caspase 3 activation were examined using immunohistochemistry. Dendritic plasticity, cleavvage-dependent activation of calcineurin A and glial fibrillary acidic protein cleavage were analyzed by immunoblotting. We found that kainic acid not only induced neurodegeneration but also arouse several caspase 3-mediated molecular and cellular changes including dendritic plasticity, neurogenesis, and gliosis. The acute caspase 3 activation occurred in pyramidal neurons as well as in hilar interneurons. The delayed caspase 3 activation occurred in astrocytes. The co-injection of caspase 3 inhibitor did not rescue kainic acid-mediated neurodegeneration but seriously and reversibly disturb the structural integrity of axon and dendrite. The kainic acid-induced events include microglia activation, the proliferation of radial glial cells, neurogenesis, and calcineurin A cleavage were significantly inhibited by the co-injection of caspase 3 inhibitor, suggesting the direct involvement of caspase 3 in these events. Alternatively, the kainic acid-mediated astrogliosis is not caspase 3-dependent, although caspase 3 cleavage of glial fibrillary acidic protein occurred.
Conclusions: Our results provide the first direct evidence of a causal role of caspase 3 activation in the cellular changes during kainic acid-mediated excitotoxicity. These findings may highlight novel pharmacological strategies to arrest disease progression and control seizures that are refractory to classical anticonvulsant treatment.
Figures





References
-
- McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;20:re12. - PubMed
-
- Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;20(Suppl 2):13–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

