Qualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPD
- PMID: 24314123
- PMCID: PMC4029771
- DOI: 10.1186/1471-2466-13-72
Qualitative assessment of attributes and ease of use of the ELLIPTA™ dry powder inhaler for delivery of maintenance therapy for asthma and COPD
Abstract
Background: Medications for respiratory disorders including asthma and chronic obstructive pulmonary disease (COPD) are typically delivered to the lung by means of a handheld inhaler. Patient preference for and ability to use the inhaler may influence their adherence to maintenance therapy, and adherence may affect treatment outcomes. In this study, patient experience of using a dry powder inhaler (DPI), the ELLIPTA™ DPI, in clinical trials of a new maintenance therapy for asthma and COPD was investigated. The ELLIPTA DPI has been designed to contain two separate blister strips from which inhalation powder can be delivered, and to be simple to use with a large, easy-to-read dose counter.
Methods: Semi-structured, in-depth, qualitative interviews were carried out 2-4 weeks after patients had completed one of six phase IIIa clinical trials using the ELLIPTA DPI. Interview participants were asked about their satisfaction with various attributes of the inhaler and their preference for the ELLIPTA DPI relative to currently-prescribed inhalers, and responses were explored using an inductive content analysis approach. Participants also rated the performance of the inhaler on several criteria, using a subjective 1-10 scale.
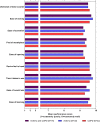
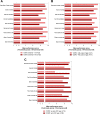
Results: Participants with asthma (n = 33) and COPD (n = 42) reported high levels of satisfaction with the ELLIPTA DPI. It was frequently described as straightforward to operate and easy to use by interview participants. Ergonomic design, mouthpiece fit, and dose counter visibility and ease of interpretation emerged as frequently cited drivers of preference for the ELLIPTA DPI compared with their current prescribed inhaler. Of participants with asthma, 71% preferred the ELLIPTA DPI to DISKUS™ and 60% to metered dose inhalers. Of participants with COPD, 86% preferred the ELLIPTA DPI to DISKUS, 95% to HandiHaler™, and 85% to metered dose inhalers. Overall average performance scores were >9 (out of 10) in participants with asthma and COPD.
Conclusion: The ELLIPTA DPI was associated with high patient satisfaction and was preferred to other inhalers by interview participants with asthma and COPD. The development of an inhaler that is regarded as easy and intuitive to use may have positive implications for adherence to therapy in asthma and COPD.
Trial registration: ClinicalTrials.gov NCT01009463 NCT01017952 NCT01053988 NCT01054885 NCT01165138 NCT01431950.
Figures

References
-
- Bender BG, Rand C. Medication non-adherence and asthma treatment cost. Curr Opin Allergy Clin Immunol. 2004;13:191–195. - PubMed
-
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention Updated 2012. Available at: http://www.ginasthma.org/guidelines-gina-report-global-strategy-for-asth.... Accessed: 2 October 2013.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

