A role for correlated spontaneous activity in the assembly of neural circuits
- PMID: 24314725
- PMCID: PMC4560201
- DOI: 10.1016/j.neuron.2013.10.030
A role for correlated spontaneous activity in the assembly of neural circuits
Erratum in
- Neuron. 2014 Jan 8;81(1):218
Abstract
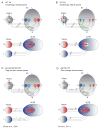
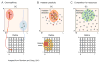
Before the onset of sensory transduction, developing neural circuits spontaneously generate correlated activity in distinct spatial and temporal patterns. During this period of patterned activity, sensory maps develop and initial coarse connections are refined, which are critical steps in the establishment of adult neural circuits. Over the last decade, there has been substantial evidence that altering the pattern of spontaneous activity disrupts refinement, but the mechanistic understanding of this process remains incomplete. In this review, we discuss recent experimental and theoretical progress toward the process of activity-dependent refinement, focusing on circuits in the visual, auditory, and motor systems. Although many outstanding questions remain, the combination of several novel approaches has brought us closer to a comprehensive understanding of how complex neural circuits are established by patterned spontaneous activity during development.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
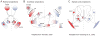



References
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci. 2000;20:7672–7681. - PMC - PubMed
-
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

