A modular gain-of-function approach to generate cortical interneuron subtypes from ES cells
- PMID: 24314726
- PMCID: PMC5085060
- DOI: 10.1016/j.neuron.2013.09.022
A modular gain-of-function approach to generate cortical interneuron subtypes from ES cells
Abstract
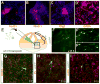
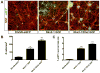
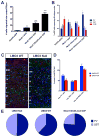
Whereas past work indicates that cortical interneurons (cINs) can be generically produced from stem cells, generating large numbers of specific subtypes of this population has remained elusive. This reflects an information gap in our understanding of the transcriptional programs required for different interneuron subtypes. Here, we have utilized the directed differentiation of stem cells into specific subpopulations of cortical interneurons as a means to identify some of these missing factors. To establish this approach, we utilized two factors known to be required for the generation of cINs, Nkx2-1 and Dlx2. As predicted, their regulated transient expression greatly improved the differentiation efficiency and specificity over baseline. We extended upon this "cIN-primed" model in order to establish a modular system whereby a third transcription factor could be systematically introduced. Using this approach, we identified Lmo3 and Pou3f4 as genes that can augment the differentiation and/or subtype specificity of cINs in vitro.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science (New York, NY. 1997;278:474–476. - PubMed
-
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. - PubMed
-
- Andrews GL, Yun K, Rubenstein JL, Mastick GS. Dlx transcription factors regulate differentiation of dopaminergic neurons of the ventral thalamus. Molecular and cellular neurosciences. 2003;23:107–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

