The THO complex regulates pluripotency gene mRNA export and controls embryonic stem cell self-renewal and somatic cell reprogramming
- PMID: 24315442
- PMCID: PMC3962795
- DOI: 10.1016/j.stem.2013.10.008
The THO complex regulates pluripotency gene mRNA export and controls embryonic stem cell self-renewal and somatic cell reprogramming
Abstract
Embryonic stem cell (ESC) self-renewal and differentiation are governed by a broad-ranging regulatory network. Although the transcriptional regulatory mechanisms involved have been investigated extensively, posttranscriptional regulation is still poorly understood. Here we describe a critical role of the THO complex in ESC self-renewal and differentiation. We show that THO preferentially interacts with pluripotency gene transcripts through Thoc5 and is required for self-renewal at least in part by regulating their export and expression. During differentiation, THO loses its interaction with those transcripts due to reduced Thoc5 expression, leading to decreased expression of pluripotency proteins that facilitates exit from self-renewal. THO is also important for the establishment of pluripotency, because its depletion inhibits somatic cell reprogramming and blastocyst development. Together, our data indicate that THO regulates pluripotency gene mRNA export to control ESC self-renewal and differentiation, and therefore uncover a role for this aspect of posttranscriptional regulation in stem cell fate specification.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
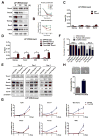
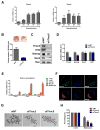

Comment in
-
Export and expression: mRNAs deliver new messages for controlling pluripotency.Cell Stem Cell. 2014 May 1;14(5):549-50. doi: 10.1016/j.stem.2014.04.009. Cell Stem Cell. 2014. PMID: 24792108
References
-
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. - PubMed
-
- Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

