Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders
- PMID: 24315484
- PMCID: PMC3897394
- DOI: 10.1016/j.cell.2013.11.024
Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders
Abstract
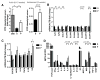
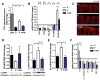
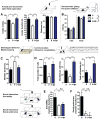
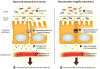
Neurodevelopmental disorders, including autism spectrum disorder (ASD), are defined by core behavioral impairments; however, subsets of individuals display a spectrum of gastrointestinal (GI) abnormalities. We demonstrate GI barrier defects and microbiota alterations in the maternal immune activation (MIA) mouse model that is known to display features of ASD. Oral treatment of MIA offspring with the human commensal Bacteroides fragilis corrects gut permeability, alters microbial composition, and ameliorates defects in communicative, stereotypic, anxiety-like and sensorimotor behaviors. MIA offspring display an altered serum metabolomic profile, and B. fragilis modulates levels of several metabolites. Treating naive mice with a metabolite that is increased by MIA and restored by B. fragilis causes certain behavioral abnormalities, suggesting that gut bacterial effects on the host metabolome impact behavior. Taken together, these findings support a gut-microbiome-brain connection in a mouse model of ASD and identify a potential probiotic therapy for GI and particular behavioral symptoms in human neurodevelopmental disorders.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Toward effective probiotics for autism and other neurodevelopmental disorders.Cell. 2013 Dec 19;155(7):1446-8. doi: 10.1016/j.cell.2013.11.035. Cell. 2013. PMID: 24360269 Free PMC article.
-
Microbiome: B. fragilis and the brain.Nat Rev Microbiol. 2014 Feb;12(2):76-7. doi: 10.1038/nrmicro3197. Epub 2013 Dec 23. Nat Rev Microbiol. 2014. PMID: 24362469 No abstract available.
-
Neurodevelopmental disorders: human gut microbiota alleviate behavioural symptoms in a mouse model of autism spectrum disorder.Nat Rev Neurol. 2014 Feb;10(2):60. doi: 10.1038/nrneurol.2013.267. Epub 2013 Dec 24. Nat Rev Neurol. 2014. PMID: 24366270 No abstract available.
-
Neurodevelopmental disorders: the gut-microbiome-brain connection.Nat Rev Neurosci. 2014 Feb;15(2):65. doi: 10.1038/nrn3669. Epub 2013 Dec 27. Nat Rev Neurosci. 2014. PMID: 24370876 No abstract available.
-
Neurodevelopmental disorders: The gut-microbiome-brain connection.Nat Rev Drug Discov. 2014 Feb;13(2):104. doi: 10.1038/nrd4235. Nat Rev Drug Discov. 2014. PMID: 24481309 No abstract available.
-
Gut microbiota and brain function: a new target for brain diseases?CNS Neurol Disord Drug Targets. 2014;13(5):733-5. doi: 10.2174/187152731305140717104045. CNS Neurol Disord Drug Targets. 2014. PMID: 24804685 No abstract available.
References
-
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid inflammatory cytokines: Potential markers of immunologic dysfunction in autism spectrum disorders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14:528–538. - PubMed
-
- Altieri L, Neri C, Sacco R, Curatolo P, Benvenuto A, Muratori F, Santocchi E, Bravaccio C, Lenti C, Saccani M, et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. 2011;16:252–260. - PubMed
-
- Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. - PubMed
-
- Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

