Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem
- PMID: 24315728
- PMCID: PMC3946275
- DOI: 10.1016/j.neurobiolaging.2013.11.006
Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem
Abstract
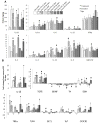
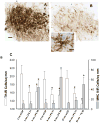
Neuroinflammation and degeneration of ascending catecholaminergic systems occur early in the neurodegenerative process. Age and the duration of a pro-inflammatory environment induced by continuous intraventricular lipopolysaccharide (LPS) differentially affect the expression profile of pro- and anti-inflammatory genes and proteins as well as the number of activated microglia (express major histocompatibility complex II; MHC II) and the integrity and density of ascending catecholaminergic neural systems originating from the locus coeruleus (LC) and substantia nigra pars compacta (SNpc) in rats. LPS infusion increased gene expression and/or protein levels for both pro- and anti-inflammatory biomarkers. Although LPS infusion stimulated a robust increase in IL-1ß gene and protein expression, this increase was blunted with age. LPS infusion also increased the density of activated microglia cells throughout the midbrain and brainstem. Corresponding to the development of a pro-inflammatory environment, LC and SNpc neurons immunopositive for tyrosine-hydroxylase (the rate-limiting synthetic enzyme for dopamine and norepinephrine) decreased in number, along with a decrease in tyrosine-hydroxylase gene expression in the midbrain and/or brainstem region. Our data support the concept that continuous exposure to a pro-inflammatory environment drives exaggerated changes in the production and release of inflammatory mediators that interact with age to impair functional capacity of the SNpc and LC.
Keywords: Aging; Alzheimer's disease; Cytokines; Locus coeruleus; Microglia; Neuroinflammation; Parkinson's disease; Rat; Substantia nigra.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
The authors declare no conflicts of interest.
Figures

References
-
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease: a community based, prospective study. Neurol. 2001;56:730–736. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cooper NR, Eikelenboom P, Emmerling M, Fiebich B, Finch CE, Frautschy S, Griffin WS, Hampel H, Landreth G, McGeer PL, Mrak R, MacKenzie I, O’Banion K, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray A. Inflammation in Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Bartels AL, Leenders KL. Neuroinflammation in the pathophysiology of Parkinson’s disease: evidence from animal models to human in vivo studies with [11C] PK11195 PET. Mov Disord. 2005;22:1852–1856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

