Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats
- PMID: 24316035
- PMCID: PMC4072042
- DOI: 10.1016/j.exger.2013.11.011
Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats
Abstract
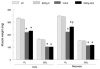
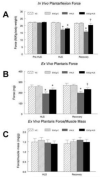
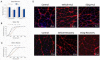
Aging exacerbates muscle loss and slows the recovery of muscle mass and function after disuse. In this study we investigated the potential that epigallocatechin-3-gallate (EGCg), an abundant catechin in green tea, would reduce signaling for apoptosis and promote skeletal muscle recovery in the fast plantaris muscle and the slow soleus muscle after hindlimb suspension (HLS) in senescent animals. Fischer 344 × Brown Norway inbred rats (age 34 months) received either EGCg (50 mg/kg body weight), or water daily by gavage. One group of animals received HLS for 14 days and a second group of rats received 14 days of HLS, then the HLS was removed and they recovered from this forced disuse for 2 weeks. Animals that received EGCg over the HLS followed by 14 days of recovery, had a 14% greater plantaris muscle weight (p<0.05) as compared to the animals treated with the vehicle over this same period. Plantaris fiber area was greater after recovery in EGCg (2715.2±113.8 μm(2)) vs. vehicle treated animals (1953.0±41.9 μm(2)). In addition, activation of myogenic progenitor cells was improved with EGCg over vehicle treatment (7.5% vs. 6.2%) in the recovery animals. Compared to vehicle treatment, the apoptotic index was lower (0.24% vs. 0.52%), and the abundance of pro-apoptotic proteins Bax (-22%), and FADD (-77%) was lower in EGCg treated plantaris muscles after recovery. While EGCg did not prevent unloading-induced atrophy, it improved muscle recovery after the atrophic stimulus in fast plantaris muscles. However, this effect was muscle specific because EGCg had no major impact in reversing HLS-induced atrophy in the slow soleus muscle of old rats.
Keywords: Apoptosis; Catechin; Muscle atrophy; Muscle fiber area; Muscle function; Sarcopenia.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures








References
-
- Adams GR. Satellite cell proliferation and skeletal muscle hypertrophy. Appl.Physiol Nutr.Metab. 2006;31:782–790. - PubMed
-
- Alway SE. Force and contractile characteristics after stretch overload in quail anterior latissimus dorsi muscle. J.Appl.Physiol. 1994;77:135–141. - PubMed
-
- Alway SE. Attenuation of Ca(2+)-activated ATPase and shortening velocity in hypertrophied fast twitch skeletal muscle from aged Japanese quail. Exp.Gerontol. 2002;37:665–678. - PubMed
-
- Alway SE, Lowe DA, Chen KD. The effects of age and hindlimb supension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp.Physiol. 2001;86:509–517. - PubMed
-
- Alway SE, Martyn JK, Ouyang J, Chaudhrai A, Murlasits ZS. Id2 expression during apoptosis and satellite cell activation in unloaded and loaded quail skeletal muscles. Am.J.Physiol Regul.Integr.Comp Physiol. 2003;284:R540–R549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

