Bacterial expression of human kynurenine 3-monooxygenase: solubility, activity, purification
- PMID: 24316190
- PMCID: PMC3969302
- DOI: 10.1016/j.pep.2013.11.015
Bacterial expression of human kynurenine 3-monooxygenase: solubility, activity, purification
Abstract
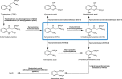
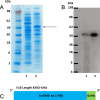
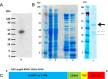
Kynurenine 3-monooxygenase (KMO) is an enzyme central to the kynurenine pathway of tryptophan metabolism. KMO has been implicated as a therapeutic target in several disease states, including Huntington's disease. Recombinant human KMO protein production is challenging due to the presence of transmembrane domains, which localise KMO to the outer mitochondrial membrane and render KMO insoluble in many in vitro expression systems. Efficient bacterial expression of human KMO would accelerate drug development of KMO inhibitors but until now this has not been achieved. Here we report the first successful bacterial (Escherichia coli) expression of active FLAG™-tagged human KMO enzyme expressed in the soluble fraction and progress towards its purification.
Keywords: 3-Hydroxykynurenine; Kynurenine 3-monooxygenase; Purification; Solubility.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Vecsei L., Szalardy L., Fulop F., Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discov. 2012;12(1):64–82. - PubMed
-
- Moffett J.R., Namboodiri M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003;81:247–265. - PubMed
-
- Okuda S., Nishiyamam N., Saito H., Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998;70:299–307. - PubMed
-
- Logters T.T., Laryea M.D., Altricher J., Sokolowski J., Cinatl J., Reipen J., Linhart W., Windolf J., Sholz M., Wild M. Increased plasma kynurenine values and kynurenine-tryptophan ratios after major trauma are early indicators for the development of sepsis. Shock. 2009;32(1):29–34. - PubMed
-
- Mole D.J., McFerran N.V., Collett G., O’Neill C., Diamond T., Garden O.J., Kylanpaa L., Repo H., Deitch E.A. Tryptophan catabolites in mesenteric lymph may contribute to pancreatitis-associated organ failure. Br. J. Surg. 2008;95:855–867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

