Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone
- PMID: 24316418
- PMCID: PMC4358819
- DOI: 10.1016/j.bone.2013.11.026
Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone
Abstract
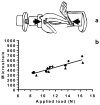
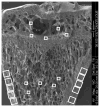
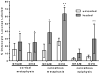
To test if osteoporosis alters mechanical load-induced interstitial fluid flow in bone, this study examined the combined effect of estrogen deficiency and external loading on solute transport around osteocytes. An in vivo tracer, FITC-labeled bovine serum albumin, was injected into anesthetized ovariectomized and control female Sprague-Dawley rats before the right tibia was subjected to a controlled, physiological, non-invasive sinusoidal load to mimic walking. Tracer movement through the lacunar-canalicular system surrounding osteocytes was quantified in cortical and cancellous bone from the proximal tibia using confocal microscopy, with the non-loaded tibia serving as internal control. Overall, the application of mechanical loading increased the percentage of osteocyte lacunae labeled with injected tracer, and ovariectomy further enhanced movement of tracer. An analysis of separate regions demonstrated that ovariectomy enhanced in vivo transport of the injected tracer in the cancellous bone of the tibial epiphysis and metaphysis but not in the cortical bone of the metaphysis. These findings show that bone changes due to reduced estrogen levels alter convectional transport around osteocytes in cancellous bone and demonstrate a functional difference of interstitial fluid flow around osteocytes in estrogen-deficient rats undergoing the same physical activity as controls. The altered interstitial fluid flow around osteocytes is likely related to nanostructural matrix-mineral level differences recently demonstrated at the lacunar-canalicular surface of estrogen-deficient rats, which could affect the transmission of mechanical loads to the osteocyte.
Keywords: Canaliculi; Interstitial fluid; Mechanotransduction; Osteocyte; Osteoporosis; Perilacunar remodeling.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


References
-
- Hancox NM. Biology of Bone. Cambridge, England: Cambridge University Press; 1972.
-
- Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. J Biomech Eng. 1991;113:191–7. - PubMed
-
- Lanyon LE. Osteocytes, strain detection, bone modeling and remodeling. Calcif Tissue Int. 1993;53 (Suppl 1):S102–6. - PubMed
-
- Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30:693–702. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

