A tetra(ethylene glycol) derivative of benzothiazole aniline ameliorates dendritic spine density and cognitive function in a mouse model of Alzheimer's disease
- PMID: 24316432
- PMCID: PMC4861143
- DOI: 10.1016/j.expneurol.2013.11.023
A tetra(ethylene glycol) derivative of benzothiazole aniline ameliorates dendritic spine density and cognitive function in a mouse model of Alzheimer's disease
Abstract
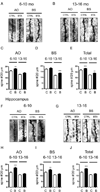
We recently reported that the tetra(ethylene glycol) derivative of benzothiazole aniline, BTA-EG4, acts as an amyloid-binding small molecule that promotes dendritic spine density and cognitive function in wild-type mice. This raised the possibility that BTA-EG4 may benefit the functional decline seen in Alzheimer's disease (AD). In the present study, we directly tested whether BTA-EG4 improves dendritic spine density and cognitive function in a well-established mouse model of AD carrying mutations in APP, PS1 and tau (APPswe;PS1M146V;tauP301L, 3xTg AD mice). We found that daily injections of BTA-EG4 for 2 weeks improved dendritic spine density and cognitive function of 3xTg AD mice in an age-dependent manner. Specifically, BTA-EG4 promoted both dendritic spine density and morphology alterations in cortical layers II/III and in the hippocampus at 6-10 months of age compared to vehicle-injected mice. However, at 13-16 months of age, only cortical spine density was improved without changes in spine morphology. The changes in dendritic spine density correlated with Ras activity, such that 6-10 month old BTA-EG4 injected 3xTg AD mice had increased Ras activity in the cortex and hippocampus, while 13-16 month old mice only trended toward an increase in Ras activity in the cortex. Finally, BTA-EG4 injected 3xTg AD mice at 6-10 months of age showed improved learning and memory; however, only minimal improvement was observed at 13-16 months of age. This behavioral improvement corresponds to a decrease in soluble Aβ 40 levels. Taken together, these findings suggest that BTA-EG4 may be beneficial in ameliorating the synaptic loss seen in early AD.
Keywords: 3xTg AD mice; AD; Alzheimer's disease; Aβ; BTA-EG(4); Dendritic spine; Ras signaling; amyloid-β.
Copyright © 2013. Published by Elsevier Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

