HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection
- PMID: 24316494
- PMCID: PMC4062949
- DOI: 10.1016/j.semcancer.2013.11.003
HTLV-1 clonality in adult T-cell leukaemia and non-malignant HTLV-1 infection
Abstract
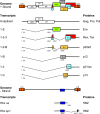
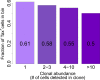
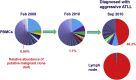
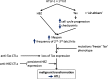
Human T lymphotropic virus type 1 (HTLV-1) causes a range of chronic inflammatory diseases and an aggressive malignancy of T lymphocytes known as adult T-cell leukaemia/lymphoma (ATLL). A cardinal feature of HTLV-1 infection is the presence of expanded clones of HTLV-1-infected T cells, which may persist for decades. A high viral burden (proviral load) is associated with both the inflammatory and malignant diseases caused by HTLV-1, and it has been believed that the oligoclonal expansion of infected cells predisposes to these diseases. However, it is not understood what regulates the clonality of HTLV-1 in vivo, that is, the number and abundance of HTLV-1-infected T cell clones. We review recent advances in the understanding of HTLV-1 infection and disease that have come from high-throughput quantification and analysis of HTLV-1 clonality in natural infection.
Keywords: Clonality; HTLV-1; High-throughput sequencing; Integration; Leukaemia; Lymphoma; Persistent infection; Retrovirus.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Nakagawa M., Izumo S., Ijichi S., Kubota H., Arimura K., Kawabata M. HTLV-I-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995;1:50–61. - PubMed
-
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. - PubMed
-
- Gallo R.C. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24:5926–5930. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

