Pantethine treatment is effective in recovering the disease phenotype induced by ketogenic diet in a pantothenate kinase-associated neurodegeneration mouse model
- PMID: 24316510
- PMCID: PMC3891449
- DOI: 10.1093/brain/awt325
Pantethine treatment is effective in recovering the disease phenotype induced by ketogenic diet in a pantothenate kinase-associated neurodegeneration mouse model
Abstract
Pantothenate kinase-associated neurodegeneration, caused by mutations in the PANK2 gene, is an autosomal recessive disorder characterized by dystonia, dysarthria, rigidity, pigmentary retinal degeneration and brain iron accumulation. PANK2 encodes the mitochondrial enzyme pantothenate kinase type 2, responsible for the phosphorylation of pantothenate or vitamin B5 in the biosynthesis of co-enzyme A. A Pank2 knockout (Pank2(-/-)) mouse model did not recapitulate the human disease but showed azoospermia and mitochondrial dysfunctions. We challenged this mouse model with a low glucose and high lipid content diet (ketogenic diet) to stimulate lipid use by mitochondrial beta-oxidation. In the presence of a shortage of co-enzyme A, this diet could evoke a general impairment of bioenergetic metabolism. Only Pank2(-/-) mice fed with a ketogenic diet developed a pantothenate kinase-associated neurodegeneration-like syndrome characterized by severe motor dysfunction, neurodegeneration and severely altered mitochondria in the central and peripheral nervous systems. These mice also showed structural alteration of muscle morphology, which was comparable with that observed in a patient with pantothenate kinase-associated neurodegeneration. We here demonstrate that pantethine administration can prevent the onset of the neuromuscular phenotype in mice suggesting the possibility of experimental treatment in patients with pantothenate kinase-associated neurodegeneration.
Keywords: ketogenic diet; mitochondria; pantethine; pantothenate kinase-associated neurodegeneration (PKAN).
Figures

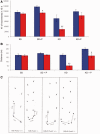

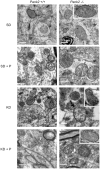
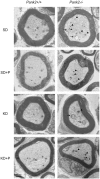
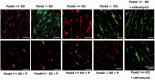
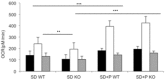

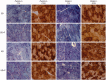
Comment in
-
The synthesis of minds and molecules leads to potential therapy for pantothenate kinase-associated neurodegeneration.Brain. 2014 Jan;137(Pt 1):8-11. doi: 10.1093/brain/awt351. Brain. 2014. PMID: 24424915 No abstract available.
Similar articles
-
The synthesis of minds and molecules leads to potential therapy for pantothenate kinase-associated neurodegeneration.Brain. 2014 Jan;137(Pt 1):8-11. doi: 10.1093/brain/awt351. Brain. 2014. PMID: 24424915 No abstract available.
-
Pantothenate kinase-associated neurodegeneration: altered mitochondria membrane potential and defective respiration in Pank2 knock-out mouse model.Hum Mol Genet. 2012 Dec 15;21(24):5294-305. doi: 10.1093/hmg/dds380. Epub 2012 Sep 13. Hum Mol Genet. 2012. PMID: 22983956 Free PMC article.
-
Down regulation of the expression of mitochondrial phosphopantetheinyl-proteins in pantothenate kinase-associated neurodegeneration: pathophysiological consequences and therapeutic perspectives.Orphanet J Rare Dis. 2021 May 5;16(1):201. doi: 10.1186/s13023-021-01823-3. Orphanet J Rare Dis. 2021. PMID: 33952316 Free PMC article.
-
Rational Design of Novel Therapies for Pantothenate Kinase-Associated Neurodegeneration.Mov Disord. 2021 Sep;36(9):2005-2016. doi: 10.1002/mds.28642. Epub 2021 May 18. Mov Disord. 2021. PMID: 34002881 Review.
-
Atypical pantothenate kinase-associated neurodegeneration with PANK2 mutations : clinical description and a review of the literature.Neurocase. 2020 Jun;26(3):175-182. doi: 10.1080/13554794.2020.1752739. Epub 2020 Apr 20. Neurocase. 2020. PMID: 32310012 Review.
Cited by
-
Pantothenate kinase 2 interacts with PINK1 to regulate mitochondrial quality control via acetyl-CoA metabolism.Nat Commun. 2022 May 3;13(1):2412. doi: 10.1038/s41467-022-30178-x. Nat Commun. 2022. PMID: 35504872 Free PMC article.
-
Natural history and genotype-phenotype correlation of pantothenate kinase-associated neurodegeneration.CNS Neurosci Ther. 2020 Jul;26(7):754-761. doi: 10.1111/cns.13294. Epub 2020 Feb 11. CNS Neurosci Ther. 2020. PMID: 32043823 Free PMC article. Review.
-
Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and α-Lipoic Acid.Int J Mol Sci. 2021 Jan 2;22(1):403. doi: 10.3390/ijms22010403. Int J Mol Sci. 2021. PMID: 33401674 Free PMC article. Review.
-
Characterization of the Pank2-/- mouse retinal phenotype as a pre-clinical model for pantothenate kinase-associated neurodegeneration.PLoS One. 2025 Jun 24;20(6):e0326866. doi: 10.1371/journal.pone.0326866. eCollection 2025. PLoS One. 2025. PMID: 40554626 Free PMC article.
-
Induction of Neuron-Specific Degradation of Coenzyme A Models Pantothenate Kinase-Associated Neurodegeneration by Reducing Motor Coordination in Mice.PLoS One. 2015 Jun 8;10(6):e0130013. doi: 10.1371/journal.pone.0130013. eCollection 2015. PLoS One. 2015. PMID: 26052948 Free PMC article.
References
-
- Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Curr Opin Clin Nutr Metab Care. 2006;9:728–33. - PubMed
-
- Bousquet M, Gibrat C, Ouellet M, Rouillard C, Calon F, Cicchetti F. Cystamine metabolism and brain transport properties: clinical implications for neurodegenerative diseases. J Neurochem. 2010;114:1651–8. - PubMed
-
- Corden BJ, Schulman JD, Schneider JA, Thoene JG. Adverse reactions to oral cysteamine use in nephropathic cystinosis. Dev Pharmacol Ther. 1981;3:25–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

