Protonation drives the conformational switch in the multidrug transporter LmrP
- PMID: 24316739
- PMCID: PMC4749020
- DOI: 10.1038/nchembio.1408
Protonation drives the conformational switch in the multidrug transporter LmrP
Abstract
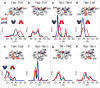
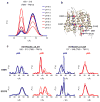
Multidrug antiporters of the major facilitator superfamily couple proton translocation to the extrusion of cytotoxic molecules. The conformational changes that underlie the transport cycle and the structural basis of coupling of these transporters have not been elucidated. Here we used extensive double electron-electron resonance measurements to uncover the conformational equilibrium of LmrP, a multidrug transporter from Lactococcus lactis, and to investigate how protons and ligands shift this equilibrium to enable transport. We find that the transporter switches between outward-open and outward-closed conformations, depending on the protonation states of specific acidic residues forming a transmembrane protonation relay. Our data can be framed in a model of transport wherein substrate binding initiates the transport cycle by opening the extracellular side. Subsequent protonation of membrane-embedded acidic residues induces substrate release to the extracellular side and triggers a cascade of conformational changes that concludes in proton release to the intracellular side.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Putman M, van Veen HW, Degener JE, Konings WN. The lactococcal secondary multidrug transporter LmrP confers resistance to lincosamides, macrolides, streptogramins and tetracyclines. Microbiology. 2001;147:2873–80. - PubMed
-
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–70. - PubMed
-
- Tanford C. Mechanism of free energy coupling in active transport. Annu Rev Biochem. 1983;52:379–409. - PubMed
-
- Forrest LR, Krämer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta. 2011;1807:167–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

