Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose
- PMID: 24317119
- PMCID: PMC3886895
- DOI: 10.1038/nm.3416
Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C-labeled glucose
Abstract
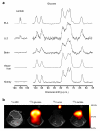
In this study, we monitored glycolysis in mouse lymphoma and lung tumors by measuring the conversion of hyperpolarized [U-2H, U-13C]glucose to lactate using 13C magnetic resonance spectroscopy and spectroscopic imaging. We observed labeled lactate only in tumors and not in surrounding normal tissue or other tissues in the body and found that it was markedly decreased at 24 h after treatment with a chemotherapeutic drug. We also detected an increase in a resonance assigned to 6-phosphogluconate in the pentose phosphate pathway. This technique could provide a new way of detecting early evidence of tumor treatment response in the clinic and of monitoring tumor pentose phosphate pathway activity.
Figures

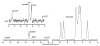
References
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Rev Cancer. 2004;4:891–899. - PubMed
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Rev Cancer. 2011;11:85–95. - PubMed
-
- Brindle K. New approaches for imaging tumour responses to treatment. Nature Rev Cancer. 2008;8:94–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

