Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial
- PMID: 24317176
- PMCID: PMC3906991
- DOI: 10.1093/jnci/djt337
Final overall survival: fulvestrant 500 mg vs 250 mg in the randomized CONFIRM trial
Abstract
Background: At the time of the initial analysis of overall survival (OS) for the Comparison of Faslodex in Recurrent or Metastatic Breast Cancer (CONFIRM) randomized, double-blind, phase III trial, approximately 50% of patients had died. A final analysis of OS was subsequently planned for when 75% of patients had died.
Methods: Patients were randomly assigned 1:1 to fulvestrant 500 mg administered as two 5-mL intramuscular injections on days 0, 14, and 28 and every 28 (±3) days thereafter or fulvestrant 250 mg administered as two 5-mL intramuscular injections (one fulvestrant and one placebo [identical in appearance to study drug]) on days 0, 14 (two placebo injections only), and 28 and every 28 (±3) days thereafter. OS was analyzed using an unadjusted log-rank test. No adjustments were made for multiplicity. Serious adverse events (SAEs) and best response to subsequent therapy were also reported. All statistical tests were two-sided.
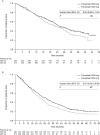
Results: In total, 736 women (median age = 61.0 years) were randomly assigned to fulvestrant 500 mg (n = 362) or 250 mg (n = 374). At the final survival analysis, 554 of 736 (75.3%) patients had died. Median OS was 26.4 months for fulvestrant 500 mg and 22.3 months for 250 mg (hazard ratio = 0.81; 95% confidence interval = 0.69-0.96; nominal P = .02). There were no clinically important differences in SAE profiles between the treatment groups; no clustering of SAEs could be detected in either treatment group. Type of first subsequent therapy and objective responses to first subsequent therapy were well balanced between the two treatment groups.
Conclusions: In patients with locally advanced or metastatic estrogen receptor-positive breast cancer, fulvestrant 500 mg is associated with a 19% reduction in risk of death and a 4.1-month difference in median OS compared with fulvestrant 250 mg. Fulvestrant 500 mg was well tolerated, and no new safety concerns were identified.
Figures
Comment in
-
RE: Final Overall Survival: Fulvestrant 500 mg vs 250 mg in the Randomized CONFIRM Trial.J Natl Cancer Inst. 2017 Mar 1;109(3):1. doi: 10.1093/jnci/djw234. J Natl Cancer Inst. 2017. PMID: 28376201 No abstract available.
References
-
- Howell A, Robertson JFR, Quaresma Albano J, et al. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20(16):3396–3403 - PubMed
-
- Cano A, Matallin P, Legua V, Tortajada M, Bonilla-Musoles F. Tamoxifen and the uterus and endometrium. Lancet. 1989;1(8634):376 - PubMed
-
- Ewer MS, Glück S. A woman’s heart: the impact of adjuvant endocrine therapy on cardiovascular health. Cancer. 2009;115(9):1813–1826 - PubMed
-
- Santen RJ. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96(2):308–319 - PubMed
-
- Osborne CK, Pippen J, Jones SE, et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20(16):3386–3395 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

